CAPCELL PAK C18 MG III, Polymer-coated C18 Stationary Phase, Designed for High-Sensitivity analyses in LC–MS
The Application Notebook
The SHISEIDO CAPCELL PAK C18 MGIII is an HPLC column packed with a silicone polymer-coated phase, providing excellent peak profiles for basic compounds under acidic conditions, and generating ultimately minimized column bleeding in LC–MS.
Kazuko Haseyama, Yoshihisa Hiroe, and Yasuo Igarashi, SHISEIDO Co., Ltd.
The SHISEIDO CAPCELL PAK C18 MGIII is an HPLC column packed with a silicone polymer-coated phase, providing excellent peak profiles for basic compounds under acidic conditions, and generating ultimately minimized column bleeding in LC–MS.
As speed and sensitivity in mass spectrometers have remarkably improved, chromatographic separations can be a major quality-determining factor in LC–MS in some cases. Often discussed are peak profiles of basic compounds under acidic mobile phases preferably used for electrospray ionization (ESI), reproducibility of retention for basic compounds under acidic conditions, and column bleeding influencing ionization efficiencies of analytes.
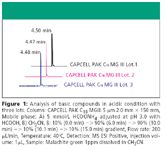
Figure 1
Since its first release in the 1990s, several different types of the CAPCELL PAK columns have been developed. The CAPCELL PAK C18 MGIII is the latest model designed to overcome the three issues above and has been developed by refining the original polymer-coating technology.
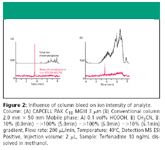
Figure 2
Experimental
CAPCELL PAK C18 MG III phase was synthesized, at first, by placing high-purity silica in contact with vapor of 1,3,5,7-tetramethylcyclo-tetrasiloxane molecules to form a reactive monolayer of the silicone polymer, and thereafter, modifying
the polymer-coated silica with 1-octadecene through hydrosilation to form C18 groups. Instruments used here were a semi-microcolumn HPLC system, NANOSPACE SI-2 (Shiseido) connected to AccuTOF, a time-of-flight mass spectrometer (JEOL, Tokyo, Japan). Other MS conditions were described in
the figures.
Results
Malachite green is a basic compound, which often induces peak tailing due to residual silanol groups on silica-based stationary phases. As shown in Figure 1, CAPCELL PAK C18 MGIII showed excellent peak shape, suggesting that the silica's undesirable secondary effect had been eliminated by the polymer-coating technology. In addition, three synthetic lots showed good reproducibility and little variation in chromatogram.
Figure 2 shows how column bleed influences the sensitivity in LC–MS. Column bleed not only messes up a total ion chromatogram, but may lower an intensity of the analyte itself. CAPCELL PAK C18 MG III showed the lowest level of column bleed among other commercially available C18 phases.
Conclusion
The polymer-coating technology resulted in an excellent C18 phase showing good peak shapes for basic compounds under acidic conditions, and also, a minimized column bleed, which seems advantageous in its applications in LC–MS.

SHISEIDO Frontier Science Business Division
1-1-16 Higashi-shimbashi, Minato-ku,
Tokyo 105-0021, Japan
Email: Chrom1@po.shiseido.co.jp

Automated Sample Preparation (ISO 20122) for MOSH/MOAH in Seasoning Oils
May 6th 2025This work presents an Automated Sample Preparation procedure for MOSH/MOAH analysis of Seasoning Oils. We compare results from a manual epoxidation procedure compliant with DIN 16995 with results based on fully automated sample preparation (epoxidation and saponification) compliant with ISO 20122. In both cases, online clean-up via activated aluminum oxide (AlOx) are used to remove interfering n-alkanes from the MOSH fraction during the HPLC run. Automated data evaluation using a dedicated software (GERSTEL ChroMOH) is presented.
Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














