Can You Use Nitrogen as an Alternate Purge Gas for Analysis of Volatile Organic Compounds (VOCs)?
OI Analytical
Laura Chambers and Gary Engelhart, OI Analytical
Most U.S. Environmental Protection Agency (U.S. EPA) methods for analysis of volatile organic compounds (VOCs) specify purging with helium for 11 min at 40 mL/min, making purge-and-trap (P&T) one of the biggest consumers of helium in a laboratory. Compared to helium, nitrogen is abundant, inert, and can be purchased at affordable prices.
This application note compares the relative response of VOCs using helium (He) and nitrogen (N2) as purge gases in U.S. EPA, P&T methods.
Experimental Conditions
Instrumentation used for this study included an OI Analytical Eclipse 4660 Purge-and-Trap Sample Concentrator equipped with an Infra-Sparge™ sample heater, a 4551A Autosampler with Standard Addition Module (SAM), and an Agilent 7890A/5975C GC–MS.
Standard P&T analytical conditions were defined as a helium purge flow rate of 40 mL/min for 11 min, the OI #10 Trap (Tenax®/silica gel/carbon molecular sieve), sample temperature of 40 °C, and a 1-min desorb time. Variables tested included the nitrogen purge gas at 40 mL/min for 11 min, OI #11 Trap (VOCARB® 3000), and modified purge and desorb times. The purge gas flow rate was measured and adjusted if necessary before each series of tests.
Results
Initial tests compared the absolute response of each compound on OI #10 and OI #11 Traps using helium and standard P&T conditions. Compound responses were comparable indicating that either trap can be used.
The next tests compared relative response of each compound on the two traps using nitrogen versus helium. With the OI #11 Trap (VOCARB®), the average relative response of some compounds varied slightly between the two purge gases, but overall performance using nitrogen was comparable to using helium (Figure 1).
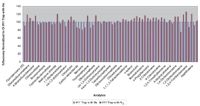
Figure 1: Relative response of 60 compounds using helium and nitrogen as the purge gas and the OI #11 Trap. Responses with helium are normalized to 100%.
Previous studies have shown that reduced purge gas volumes (for example, using shorter purge times) produce results equivalent to the standard conditions (1,2). To examine shorter purge times using nitrogen, four replicate standards were analyzed using a 7-min nitrogen purge, a 2-min desorb, and the OI #11 Trap.
Most compound responses were comparable using a 7-min nitrogen purge, and responses for the heaviest compounds improved significantly using the 2-min desorb (Figure 2).

Figure 2: Relative response of 60 compounds using an 11-min and 7-min purge with nitrogen. Responses from the 11-min nitrogen Purge are normalized to 100%.
Conclusions
This study demonstrates that nitrogen can replace helium for analysis of VOCs using P&T methods. Using nitrogen as an alternative to helium will significantly reduce the operating costs for VOC methods. The study also confirms that a 7-min purge and 2-min desorb improves analytical results while shortening overall P&T cycle time by a full 4 min on each sample.
Laboratories are advised to consult with their regulating agency prior to making modifications to P&T operating conditions.
For complete results of this study, refer to OI Application Note #3261.
References
(1) OI Analytical Application Note #3012, "Reduction of Purge–and–Trap (P&T) Cycle Times in Volatile Organic Compounds (VOCs) Analysis," 2008.
(2) Improvements to U.S. EPA Method 524.2 for the Determination of Volatile Organics, B. Prakash, Proceedings of National Environmental Monitoring Conference, August 19–25, 2007, Cambridge, Massachusetts.
OI Analytical
P.O. Box 9010, College Station, TX 77842-9010
tel. (979) 690-1711, fax (979) 690-0440
Email: oimail@oico.com Website: www.oico.com















