Application of an LC–MS/MS Library for the Identification and Quantitation of Pesticides in Food Safety Using MRM Spectrum Mode
The Column
This article describes the application of liquid chromatography tandem mass spectrometry (LC–MS/MS) and enhanced multiple reaction monitoring (MRM) spectrum libraries for multi-pesticide residue analysis. Using these methods, a high number of fragment ion transitions of target compounds can be examined to increase specificity and reporting confidence, reducing the risk of false positive or false negative detection.
Photo Credit: Nevada31/Shutterstock.com

Stéphane Moreau and Gesa Schad, Shimadzu Europa GmbH, Duisburg, Germany
This article describes the application of liquid chromatography tandem mass spectrometry (LC–MS/MS) and enhanced multiple reaction monitoring (MRM) spectrum libraries for multi-pesticide residue analysis. Using these methods, a high number of fragment ion transitions of target compounds can be examined to increase specificity and reporting confidence, reducing the risk of false positive or false negative detection.
Pesticide monitoring programmes are designed to identify and quantify pesticides within a defined regulatory framework. To minimize the risk to public health, maximum residue levels (MRLs) have been set for more than 500 pesticides in 370 food products and groups within the EU (Regulation (EC) No 396/2005). As the capability of pesticide monitoring programmes increases so too does the need for liquid chromatography tandem mass spectrometry (LC–MS/MS) methods to positively identify and quantify pesticides quickly, efficiently, and robustly. The approach described in this LC–MS/MS method brings together library searchable identification and high sensitivity quantitation to meet the needs of routine pesticide monitoring programmes.
An analytical method for the determination of 193 pesticides in a 15âmin cycle time is described. Pesticide residues were determined in several different food matrices (turmeric, plum, peppermint, parsnip, cherry, lime, pumpkin, tomato, avocado, and potato). Data was processed using an automated library search for target compounds.
Experimental
For fast and reliable screening for residual pesticides, a Nexera X2 UHPLC system was used, consisting of two solvent pumps (LC-30AD), a 20 µL solvent mixer, an autosampler (SIL-30AC), and a column oven (CTO-30A) (all Shimadzu). The system was equipped with a Shimadzu LCMSâ8060 triple quadrupole mass spectrometer via an ESI source.
UHPLC Method Parameters: Column: 100 × 2.1 mm, 1.7-µm HSS T3 (Waters); temperature: 40 °C; flow rate: 0.4 mL/min; mobile phase A: 5 mM HCOONH4 in H2O; mobile phase B: 5 mM HCOONH4 in methanol; gradient: 1.50 min at 35%B, 11.50 min at 100%B, 13.00 min at 100%B, 13.01 min at 3%B, 15.00 min stop; injection volume: 0.1 µL (+ 30 µL H2O).
Mass Spectrometry Method Parameters: Target compounds: 193; MRM transitions: spectrum mode: 1291; 2 MRM mode: 386; pause time: 1 msec; dwell time: 3 msec; ionization: ESI +/-; polarity switching: 5 msec; interface temp.: 350 °C; heat block temp.: 300 °C; desolvation line temp.: 150 °C; nebulizing gas: 3 L/min; heating gas: 10 L/min; drying gas: 10 L/min.
Fruit, vegetable, and herb samples spiked with pesticides and extracted by QuEChERSâbased methods were provided by Concept Life Sciences (UK). The method was set up using a package with more than 750 pesticides designed to accelerate method development and to help compound verification (1). To verify the applicability of the MRM spectrum mode database and library search, several matrices were tested, including turmeric, plum, peppermint, parsnip, cherry, lime, pumpkin, tomato, avocado, and potato. Final extracts in acetonitrile were used for the analysis. The peak shape of earlyâeluting compounds was improved by using a water co-injection method, performed automatically in the autosampler. For a direct comparison of the MRM spectrum mode method with conventional methods, the same method was also set up with two MRMs applied to each compound (386 MRMs in total) applying the same acquisition method described previously.
The method was calibrated in the range 0.01 mg/kg to 0.2 mg/kg and repeatability was tested using the avocado sample spiked with 0.1 mg/kg pesticide.
Fragmentation for all pesticides was optimized automatically with software (LabSolutions, Shimadzu), and an MRM spectrum mode method was generated. The MRM spectrum mode method for automated library search and compound verification was set up utilizing Shimadzu’s pesticide database containing more than 6000 MRM transitions for over 750 pesticides. Insight software was used to screen quantitative and qualitative data with advanced filtering tools to review by exception and to reduce false detect reporting.
Results
An LC–MS/MS assay for multi-pesticide residue analysis was developed to quantify and identify 193 pesticides in several food products. To increase the likelihood of a positive identification, a higher number of MRM transitions were acquired for each target compound to generate a library searchable product ion spectrum. Despite the large amount of data acquired, sensitivity was not affected by the high acquisition rate. Figure 1 shows an MRM chromatogram recorded with MRM spectrum mode, where 1291 MRM transitions were measured for 193 pesticides (0.010 mg/kg).
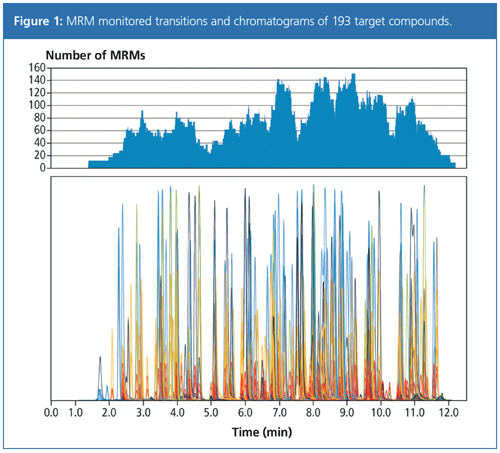
Repeatability of the method was demonstrated by the analysis of a spiked pesticide standard mixture in avocado at a concentration of 0.1 mg/kg. The highest number of overlapping MRM transitions was 151, acquired between 8.80 and 9.30 min. Even at this high data sampling rate the response correlated well with the conventional 2 MRM method with peak area variation of less than 5.2% (n = 5) for the 22 target pesticides eluted during this time period (Table 1).
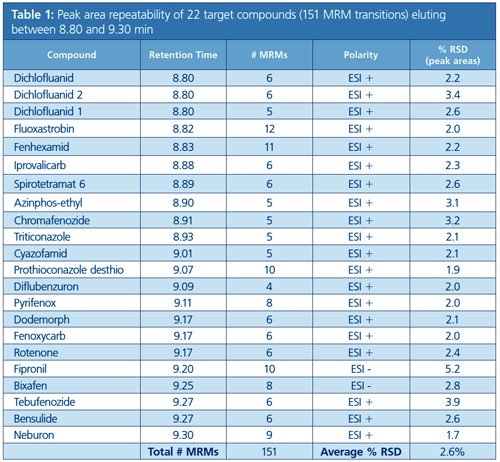
The high number of MRM transitions in the LC–MS/MS analysis of residual pesticides is designed to deliver a library searchable identification and minimize the risk of false positive or negative detection. The performance of this approach was compared with a conventional MRM method monitoring two transitions for each pesticide. Figure 2 shows a comparison of the different approaches on two example compounds, ethirimol and lufenuron, with 0.1 mg/kg spiked in avocado. The data obtained shows that regardless of the high number of fragment ions monitored, the absolute signal intensity for both methods is almost identical in positive and negative ion mode.
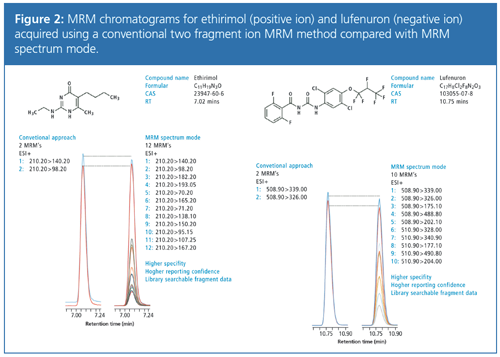
With the increased number of qualifier transitions for each analyte and resulting product ion spectra, confidence in reporting results can be significantly improved. An MRM product ion spectrum can then be compared automatically against a reference spectrum to generate a match score using conventional library searching.
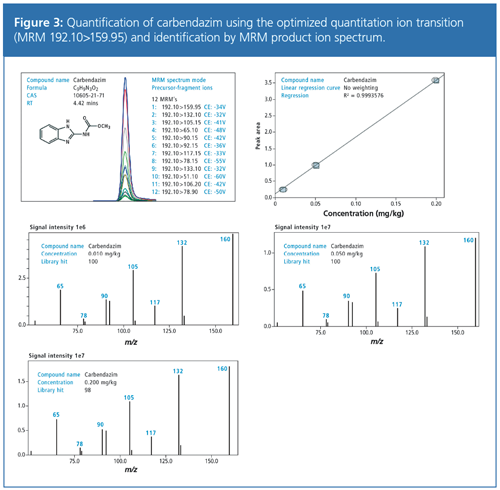
For quantitation, a target compound (for example, carbendazim) was spiked into the matrix at three different concentration levels. Figure 3 shows that all MRM transitions were detected down to the reporting level of 0.010 mg/kg with a signal-to-noise ratio for all fragment ion transitions of greater than 9. The response was linear for all transitions throughout the calibration range (0.010–0.200 mg/kg). The example compound carbendazim produced a product ion spectrum of 12 fragment ions when using collision energies between 10–60 V. The product ion spectrum could then be used for library search and analyte confirmation (Figure 3). In all library searches the similarity score was >99, providing a very high confidence in compound verification.
Conclusion
Multiple reaction monitoring (MRM)âbased LC–MS/MS methods are widely used on triple quadrupole instruments for targeted quantitation, providing high selectivity, sensitivity, and robustness. For pesticide analysis in the EU, identification criteria in SANTE/11945/2015 requires the retention time and the ion ratio from at least 2 MRM transitions to be within acceptable tolerance limits (2). But even when complying with these guidelines, it has been reported that false positives can occur in certain pesticide–commodity combinations (3–5). Use of a larger number of MRM transitions for compound identification increases the level of confidence in assay specificity. MRM spectrum mode combines conventional MRM quantitation with the generation of high quality MRM product ion spectra, which were successfully used in routine library searching and compound identification.
For each calibration level ranging from 0.010–0.200 mg/kg, the library similarity score was greater than 99, confidently confirming the target analyte. The advantage of this technique is that library searchable product ion spectrum data is used for compound identification, without compromising sensitivity, accuracy, or robustness of quantitative results.
References
- LC/MS/MS Method Package - Residual Pesticides Version 2 (P/N 225-28541) Instruction Manual
- European Commission SANTE/11945/2015. Guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed.
- A. Schürmann, V. Dvorak, C. Crüzer, P. Butcher, and A. Kaufmann, Rapid Communications in Mass Spectrometry 23(8), 1196–1200 (2009).
- A. Kaufmann, P. Butcher, K. Maden, M. Widmer, K. Giles, and D. Uría, Rapid Communications in Mass Spectrometry 23(7), 985–998 (2009).
- Ó. Pozo, J. Sancho, M. Ibáñez, F. Hernández, and W. Niessen, TrAC Trends in Analytical Chemistry25(10), 1030–1042 (2006).
Stephane Moreau obtained his diploma in fine chemistry and engineering with a specialization in chemical process engineering in 1994 from INSA (National Institute of Applied Sciences) in Rouen, France. He then started his professional career in laboratory equipment distribution before he joined the Shimadzu France subsidiary in 2002. Since then, he has held various positions to develop the MS range business. Since September 2013, he has been product manager for the MS range with Shimadzu Europe.
Gesa Johanna Schad graduated with a diploma in chemical engineering from the Technical University NTA in Isny, Germany, in 2004 and an M.Sc. in pharmaceutical analysis from the University of Strathclyde in Glasgow, UK, in 2005. She worked until 2006 as a consultant in HPLC method development and validation in an analytical laboratory of the FAO/IAEA in Vienna, Austria. She gained her doctorate for research in pharmaceutical sciences at the University of Strathclyde in 2010 and was employed as an HPLC specialist in the R&D department at Hichrom Ltd. in Reading, UK, from 2009. Since 2013, she has worked as an HPLC product specialist and since 2015 as HPLC Product Manager in the analytical business unit of Shimadzu Europa in Duisburg, Germany.
E-mail:shimadzu@shimadzu.euWebsite:www.shimadzu.eu

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.



![IMG_4773[1].jpg IMG_4773[1].jpg](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2F0vv8moc6%2Fchroma%2F6db719ea80519dad8e948ad595b96c3fa3731826-200x207.jpg%3Ffit%3Dcrop%26auto%3Dformat&w=3840&q=75)







