Investigating Cookie Aroma Profiles Using Dynamic Headspace Sampling and TD–GC–TOF-MS
The Column
This proof-of-principle study shows that dynamic headspace sampling with thermal desorption–gas chromatography–time‑of-flight mass spectrometry (TD–GC–TOF-MS) analysis can be used to investigate the complex aroma profiles released from flavoured cookies. Key flavour compounds in chocolate-chip, peanut, and orange-cream cookies are highlighted, and the effect of raising the headspace extraction temperature is examined.
Photo Credit: homydesign/Shutterstock.com

Aaron Parker, Laura McGregor, and David Barden, SepSolve Analytical, Peterborough, UK
This proof-of-principle study shows that dynamic headspace sampling with thermal desorption–gas chromatography–timeâof-flight mass spectrometry (TD–GC–TOF-MS) analysis can be used to investigate the complex aroma profiles released from flavoured cookies. Key flavour compounds in chocolate-chip, peanut, and orange-cream cookies are highlighted, and the effect of raising the headspace extraction temperature is examined.
Within the food industry, there is an increasing need to monitor product safety and quality, typically in relation to flavour composition, taint, and contamination. This requires a detailed understanding of individual components and the factors that govern their levels in the final product. This is particularly the case for baked products, such as cookies and crackers, which can be highly challenging to study because of the complex mixture of compounds produced by the baking process (1).
To tackle this challenge, various techniques are available to extract the aroma-active compounds from food matrices (2), and these are usually coupled with gas chromatography–mass spectrometry (GC–MS). The few such studies investigating cookie aroma have generally involved solvent extraction, whereby the food sample is treated with a solvent such as hexane, and 1–2 µL of the resulting extract injected into the GC system (3,4,5,6). However, the small sample volume can result in relatively high limits of detection and reduced confidence in the detection of trace-level components.
While there are approaches to reducing matrix interference and improving detection limits, these are associated with risks. For example, clean-up methods such as solid-phase extraction (SPE) may cause key analytes to be retained or eluted during the solvent washing steps. Moreover, a particular problem with any method involving solvent is chromatographic interference from the solvent or associated impurities, as well as the potential for the more volatile compounds to be lost during the process of extraction.
Headspace methods overcome these issues and are considerably easier from a practical standpoint. Solid-phase microextraction (SPME) is widely used for extracting aroma compounds from food headspace samples (7), and indeed has been applied to cookies (8), but the small volume of sorbent material on the SPME fibre can make it difficult to achieve a high degree of sensitivity.
The above points can be addressed by using dynamic headspace sampling (DHS) onto sorbent-packed tubes using a microchamber device-an approach that has been shown to work for other food products (9,10). These sorbentâpacked tubes are then analyzed by thermal desorption (TD) preconcentration, followed by GC–MS analysis. Thermal desorption is wellâestablished as an analytical technique across a wide range of application areas (111,12), and for the case of oat biscuits has already been noted to offer greater ease of sample throughput compared to SPME (13). Furthermore, the two-stage process of analyte focusing on the sorbent tube and then on the focusing trap within the TD instrument results in analytes being focused in a narrow band of vapour, greatly improving peak shape and detection limits (12).
A final aspect of the analytical setup to consider is the detector. Quadrupole instruments are widely used in the food industry, but lack sensitivity when used in scan mode and cannot be used to screen unknowns in the more sensitive selected ion monitoring (SIM) mode. Time-of-flight (TOF) mass spectrometers can overcome these problems by offering enhanced sensitivity and collection of data across the full mass range in a single run (14), while being unaffected by the phenomenon of spectral skew that is a common occurrence in spectra acquired on quadrupole instruments used in scan mode (15). Some models can generate mass spectra that match those in (usually quadrupole-acquired) commercial spectral libraries (16,17).
This study investigates the potential of these techniques for the analysis of aroma compounds in flavoured cookies, using a microchamber device for dynamic headspace extraction onto sorbent tubes, followed by TD–GC–TOF-MS.
Experimental
Samples: Three types of cookie were purchased from a local supermarket: (a) chocolate-chip, (b) peanut, and (c) orangeâcream. The cookies were broken into pieces, and a single piece (~3 g) used for each analysis.
DHS: Instrument: Micro-Chamber/Thermal Extractor (Markes International); chamber size: 44 cm3; gas flow: nitrogen, 100 mL/min (set using flowmeter on a low-flow setting); chamber temperature: 40 °C or 80 °C; equilibration: 5 min; sampling: 30 min; sorbent tubes: stainless steel, packed with Tenax TA (Markes International).
TD: Instrument: TD100-xr (Markes International); flow path: 200 °C; dry-purge: 1 min, 50 mL/min; pre-purge: 1 min, 50 mL/min; tube desorb: 300 °C (10 min), 50 mL/min, no split; trap low: –10 °C; pre-trap fire purge: 1 min, 50 mL/min; heating rate: max.; trap high: 320 °C (3 min); trap desorb: 100:1; re-collection: onto the same tube, subsequently desorbed with a 20:1 split.
GC: Instrument: 7890 GC (Agilent Technologies); column: 20 m × 0.18 mm, 0.18-µm Rxi5sil-MS (Restek Corporation); temperature program: 35 °C (3.5 min),
3 °C/min to 120 °C (0 min), 5 °C/min to
250 °C (0 min), 10 °C/min to 330 °C (5 min).
TOF-MS: Instrument: BenchTOF-HD (Markes International); filament voltage: 1.8 V; ion source: 200 °C; transfer line: 300 °C; mass range: m/z 40–600; data rate: 4 Hz at 70 eV.
Results and Discussion
Figure 1 shows example analyses of the three types of cookies run at a split ratio of 100:1 with an extraction temperature of 40 °C, which aids more complete release of volatiles from the cookie over the 30-min sampling period than a room temperature extraction would. Six samples were run simultaneously under the same conditions, making it easier to achieve consistency in sampling conditions between runs.
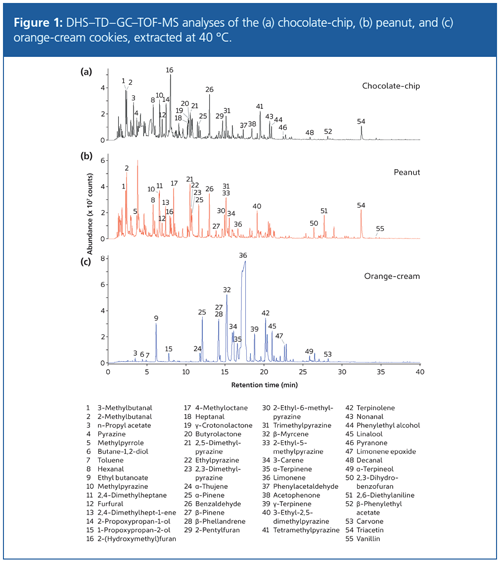
A large number of compounds were identified in the samples-only the most abundant are shown in Figure 1. Many of these are aroma-active pyrazines, alcohols, aldehydes, and ketones. Superficially, the profiles of the chocolate-chip and peanut cookies are relatively similar to each other, while the orange-cream cookie presents a substantially different profile, suggesting that the composition of the biscuit is substantially different.
Several aldehydes were present in the samples. Both the chocolate-chip and peanut cookies contained furfural (12), which is a result of pentose degradation, and is also potentially formed during the final stage of baking as a degradation product of the characteristic “browning” compound hydroxymethylfurfural (8). The same two cookies also contained benzaldehyde (26), with its well-known almond aroma, and 2- and 3-methylbutanal (3 and 2)-noted to be derived from the baking process (18) and to provide strong chocolate aromas in dark chocolates (19). Nonanal (43) and decanal (48) were found in the chocolateâchip cookie, in accordance with a report that such medium-chain aldehydes in cookies may be a consequence of lipid peroxidation at the start of baking (8).
No pyrazines were found in the orangeâcream cookie, but seven (10,22, 23,30,31,33,40) were found in the peanut cookie and four (4,10,31,41) in the chocolate-chip cookie, in agreement with the generally “nutty, roasty, earthy” aromas produced by these compounds (20).
In contrast, the citrus-derived compound limonene (36) was only present in the orangeâcream cookie (and indeed dominated this profile). The orange-cream cookie also featured several other monoterpenes, including several known as components of orange essential oil (21)-α-pinene (25), β-myrcene (32), 3-carene (34), and γ-terpinene (39). This is in accordance with the expected use of this essential oil to impart the orange flavour to these cookies.
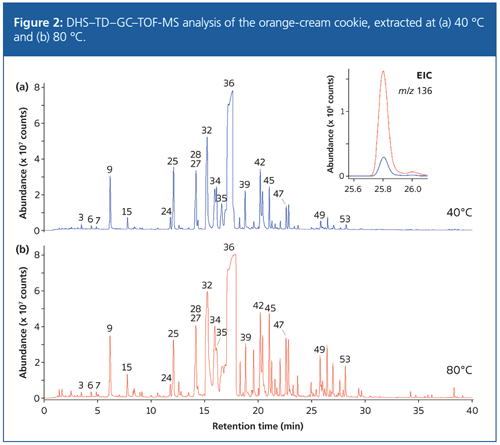
Headspace sampling conditions are expected to have a strong effect on the relative proportions of compounds extracted with different volatilities. To examine this, the chamber temperature of the microchamber device was raised to 80 °C and the headspace of the orangeâcream cookie sampled as before. The results (Figure 2) show an increase in the response from compounds across the range at the higher temperature, but especially for the higher-boiling compounds, such as α-terpineol (49). It is worth pointing out that obtaining such additional information on the sample profile is easily achievable using headspace techniques, in contrast to solventâbased procedures.
Conclusions
In this proof-of-principle study, the combination of dynamic headspace sampling with TD–GC–TOF-MS analysis is shown to offer efficient evaluation of volatile compounds from cookies, avoiding the disadvantages of solvent-based methods while enhancing sensitivity. The method should therefore be valuable for investigating aroma profiles of these and similar food products, aiding product formulation and assisting routine quality control.
References
- L.F.M. Yong, T.E. Acree, E.H. Lavin, and R.M. Butts, Thermal Generation of Aromas, T. Parliament et al., Eds. (ACS Symposium Series, 1989), chapter 26.
- J.S. Elmore, in Flavour Development, Analysis and Perception in Food and Beverages, J.K. Parker, S. Elmore, and L. Methven, Eds. (Elsevier, 2015). DOI: 10.1016/B978-1-78242-103-0.00002-3.
- M. Graf, T.M. Amrein, S. Graf, R. Szalay, F. Escheer, and R. Amadò, LWT – Food Science and Technology39, 724–728 (2006).
- K. Burseg, R.S.T. Linforth, J. Hort, and A.J. Taylor, Chemical Perceptions 2, 70–78 (2009). DOI: 10.1007/s12078-009-9042-8.
- N. Yang, I.D. Fisk, R. Linforth, K. Brown, S. Walsh, S. Mooney, C. Sturrock, and J. Hort, European Food Research Technology 235, 1083–1091 (2012). DOI: 10.1007/s00217-012-1837-1.
- N. Yan, J. Hort, R. Linforth, K. Brown, S. Walsh, and I.D. Fisk, Food Chemistry141, 1354–1360 (2013). DOI: 10.1016/j.foodchem.2013.03.084
- H.H. Jelen‘ , M. Majcher, and M. Dziadas, Analytica Chimica Acta738, 13–26 (2012). DOI: 10.1016/j.aca.2012.06.006.
- L. Ait Ameur, B. Rega, P. Giampoli, G. Trystram, and I. Birlouez-Aragon, Food Chemistry111, 758–763 (2008). DOI: 10.1016/j.foodchem.2007.12.062.
- S. Koschinski, L. McGregor, G. Roberts, and D. Barden, The Column 12(6), 16–23 (2016).
- P. Morris and D. Barden, The Column9(8), 9–13 (2013).
- J.V. Hinshaw, LCGC North America34(1), 26–32 (2016).
- Markes International Application Note, Analytical thermal desorption: History, technical aspects and application range June 2012, www.markes.com/Download-Document.aspx?GUID=46f2317f-6e4a-44fe-90a4-2538d7da67a6.
- C. Cognat, T. Shepherd, S.R. Verrall, and D. Stewart, Food Chemistry134, 1592–1600 (2012). DOI: 10.1016/j.foodchem.2012.02.119.
- J. Hoker, F. Obersteiner, H. Bönisch, and A. Engel, Atmospheric Measurement Techniques8, 2195–2206 (2015). DOI: 10.5194/amt-8-2195-2015.
- J.T. Watson and D.O. Sparkman, Introduction to Mass Spectrometry (4th edition) (John Wiley & Sons, 2007).
- D. Barden, Chromatography Techniques CT12–CT14 (2012).
- S. Smith, D. Barden, and L. McGregor, The Column 10(21), 13–17 (2014).
- M.S. Brauss, B. Balders, R.S.T. Linforth, S. Avison, and A.J. Taylor, Flavour & Fragrance Journal14, 351–357 (1999). DOI: 10.1002/(SICI)1099-1026(199911/12)14:6<351::AID-FFJ847>3.0.CO;2-L.
- C. Counet, D. Callemien, C. Ouwerx, and S. Collin, Journal of Agricultural and Food Chemistry50, 2385–2391 (2002). DOI: 10.1021/jf0114177.
- R. Wagner, M. Czerny, J. Bielorhadsky, and W. Grosch, Zeitschrift für Lebensmittel-Untersuchung und -Forschung 208, 308–316 (1999). DOI: 10.1007/s002170050422.
- A. Verzera, A. Trozzi, G. Dugo, G. Di Bella, and A. Cotroneo, Flavour and Fragrance Journal19, 544–548 (2004). DOI: 10.1002/ffj.1348.
Aaron Parker studied chemistry at the University of York and following this spent six years working within an environmental analytical laboratory, where he was responsible for developing analytical methods to accredited status for multiple analytical instruments, specializing in GC–MS. In his current position, he is focused on developing full GC and GC×GC analytical solutions, from sample preparation to reporting, as well as providing engineering support and customer training.
Laura McGregor received an M.Chem. in chemistry from the University of St Andrews, UK, followed by an M.Sc. in forensic science at the University of Strathclyde, UK. Her Ph.D. in environmental forensics, also at the University of Strathclyde, focused on the chemical fingerprinting of environmental contamination using advanced techniques such as GC×GC−TOF-MS. In her current role, she specializes in the application of GC×GC and TOF-MS to challenging applications.
David Barden studied natural sciences at the University of Cambridge, UK, and remained there for his Ph.D. in synthetic organic chemistry, which he received in 2003. A placement at Wiley-VCH, Germany, was then followed by seven years as a journals editor at Royal Society of Chemistry Publishing, UK, before beginning his current role as copywriter in 2011.
E-mail: hello@sepsolve.comWebsite:www.sepsolve.com



![IMG_4773[1].jpg IMG_4773[1].jpg](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2F0vv8moc6%2Fchroma%2F6db719ea80519dad8e948ad595b96c3fa3731826-200x207.jpg%3Ffit%3Dcrop%26auto%3Dformat&w=3840&q=75)
















Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
Using GC-MS to Measure Improvement Efforts to TNT-Contaminated Soil
April 29th 2025Researchers developing a plant microbial consortium that can repair in-situ high concentration TNT (1434 mg/kg) contaminated soil, as well as overcome the limitations of previous studies that only focused on simulated pollution, used untargeted metabolone gas chromatography-mass spectrometry (GC-MS) to measure their success.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.
Potential Obstacles in Chromatographic Analyses Distinguishing Marijuana from Hemp
April 28th 2025LCGC International's April series for National Cannabis Awareness Month concludes with a discussion with Walter B. Wilson from the National Institute of Standard and Technology’s (NIST’s) Chemical Sciences Division regarding recent research his team conducted investigating chromatographic interferences that can potentially inflate the levels of Δ9-THC in Cannabis sativa plant samples, and possible solutions to avoid this problem.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
Using GC-MS to Measure Improvement Efforts to TNT-Contaminated Soil
April 29th 2025Researchers developing a plant microbial consortium that can repair in-situ high concentration TNT (1434 mg/kg) contaminated soil, as well as overcome the limitations of previous studies that only focused on simulated pollution, used untargeted metabolone gas chromatography-mass spectrometry (GC-MS) to measure their success.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.
Potential Obstacles in Chromatographic Analyses Distinguishing Marijuana from Hemp
April 28th 2025LCGC International's April series for National Cannabis Awareness Month concludes with a discussion with Walter B. Wilson from the National Institute of Standard and Technology’s (NIST’s) Chemical Sciences Division regarding recent research his team conducted investigating chromatographic interferences that can potentially inflate the levels of Δ9-THC in Cannabis sativa plant samples, and possible solutions to avoid this problem.
2 Commerce Drive
Cranbury, NJ 08512

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)