Analyzing Aerosol Defence Pepper Spray Residues on Clothing and Analysis by GC-MS
LCGC Europe
Describes an analysis that was performed in a forensic science laboratory to assess who was the victim and who was the attacker in a legal dispute.
The use of aerosol defence pepper sprays for self-defence and as assault weapons has increased.The residue of these sprays is often left behind as physical evidence on a victim’s clothing or personal belongings. A rapid and accurate method has been developed to qualitatively determine capsaicin and its most important analogues – dihydrocapsaicin and nordihydrocapsaicin – on clothing. The methanol solvent extraction (SE) and solid-phase microextraction (SPME), recovery from fabrics, and gas chromatography mass spectrometry (GC–MS) identification of residue from aerosol defence pepper sprays are described.
Aerosol defence pepper sprays are compounds that cause temporary incapacitation by producing sensory irritation. This results in extreme discomfort (burning, sneezing, tearing, secretions, gagging, vomiting) and pain is associated with the areas affected (nose, eyes and respiratory tract). The effects begin within 3–15 s and can last 15–90 min.

The three basic components of aerosol defence pepper sprays are: the active ingredient — the irritant oleoresin capsicum (OC) at a concentration range of 0.5–20% — with a carrier and propellant. The carrier acts as a vehicle in which the irritant is suspended or dissolved. Propellants are used to expel the irritant from the canister. The sprays can be expelled with fine stream or misty cone patterns (ranges 2–6 m). The stream can be a liquid, a gel or a foam. Commonly used carriers and propellants include nitrogen, carbon dioxide, propane, butane, ethanol, propylene glycol, secondary butanol, isopropanol, tetrafluoroethane, kerosene, d-limonene, acetone, methylene chloride or 1,1,1-trichloroethane. Sometimes UV/vis dyes can be present or blends with chloroacetophenone (CN) or chlorobenzalmalononitrile (CS) are possible. Figure 1 illustrates a schematic representation of an aerosol defence pepper spray canister.
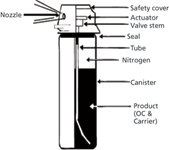
Figure 1: Schematic diagram of an aerosol defence pepper spray canister.
OC is classified as an inflammatory agent and is the primary compound of interest in this paper. Exposure to OC produces inflammation and swelling of the mucous membranes associated with the eyes, nose and throat. OC is a reddish-brown oily liquid derived from the plants of the genus Capsicum annuum L. and Capsicum frutescens L., which are commonly referred to as hot peppers or chillies.
OC contains a group of compounds called capsaicinoids (CAPs), which are responsible for the pungency associated with cayenne and other varieties of peppers. CAPs are the pharmacologically active and pain-producing components of the hot pepper. There are five naturally occurring CAPs: capsaicin, nordihydrocapsaicin, dihydrocapsaicin, homocapsaicin and homodihydrocapsaicin (the active ingredients responsible for the irritation properties are capsaicin and dihydrocapsaicin; they make up to 80–90% of the total). The exact chemical composition of OC varies with the type of pepper, its age, and parts of the plant from which the extract is obtained. Capsaicin is also widely used in food preparation as a herpes treatment and a deterrent to dog fights and swine cannibalism.
Capaiscin can also be synthesized in the laboratory. OC contains over 100 distinct volatile components in addition to capsaicin.

Table 1
The burning capacity is directly related to the concentration of capsaicin and it is evaluated by means of the organoleptic Scoville heat units (SHU) (Table 1). The percentage of capsaicin can, therefore, be converted to SHU with the following equation:
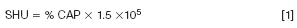
For example, a pepper spray that indicates "10% OC @ SHU 2.000.000" is calculated as [200,0000 × 0.10 ] = 200,000 SHU are released at the nozzle when spraying.
The basic chemical structure of capsaicin and its analogues is a vanilloid ring connected to an amino alkyl chain containing 9–11 carbon atoms. Figure 2 shows the five major CAPs.
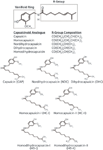
Figure 2: Five major capsaicinoids.
Pure capsaicin is insoluble in water (0.090 g at 37 °C) but soluble in oils and some solvents (alcohols, ethers, ketones, chloroform). Capsaicin forms crystals, has a melting point of 65 °C and a boiling point at 210–220 °C. It has an extrapolated vapour pressure of 1.5 × 10–7 mm Hg at 65 °C and a vapour density (relative to air) of 10.5. It exerts its effect on the sensory nerves by interacting with the vanilloid receptor, promoting the release of substance P, as well as other cytokines. The release of these cytokines from the peripheral sensory neurons causes a sensation of intense burning and pain.
News reports often show criminal actions or law enforcement abuse with aerosol defence pepper sprays. In Belgium aerosol defence pepper sprays are classified as a prohibited weapon, therefore, possession by non-police members is illegal. The extraction and identification of these compounds has become increasingly important in forensic casework. Inadequate extraction may lead to a false negative result. Commonly used extractions methods are solvent extraction (SE) and solid-phase microextraction (SPME). Identification can be performed by gas chromatography–mass spectrometry (GC–MS) or liquid chromatography–mass spectrometry (LC–MS) (1–2).
Materials and Methods
Aerosol defence pepper spray canisters were obtained at various police departments in Brussels and Ghent. Table 2 summarizes the analytical conditions we developed based on references (3) and (6–7).

Table 2: Mass ions of capsaicinoids.
The methanol solvent was of analytical grade and purchased from VWR (Heverlee, Belgium). Nitrogen for evaporating the extraction liquid was purchased from Messer (Zwijndrecht, Belgium).
Glass Pasteur pipettes, GC syringes, GC septa, glass jars with metal screw caps and glass vials with screw caps were purchased from VWR (Heverlee, Belgium). Polyethylene bags, nylon-11 bags and solvent free aluminum tape were purchased from Polis-Service / BVDA (Lille, Belgium) and RajaPAC (Tongeren, Belgium). The SPME holder and fibre (50/30 μm divinylbenzene/carboxen/polydimethylsiloxane) were purchased from Supelco (Bornem, Belgium).
Analysis was performed using a Thermo GCTraceMS+ (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Separation of the analytes was achieved using a Rtx-5MS column, 30 m × 0.32 mm i.d., 1 μm column (Restek, Bellefonte, Pennsylvania, USA). The injection volumes on the gas chromatograph were 1 μL performed in splitless mode (1 min at 260 °C) and 1 mL performed in split mode (30 mL/min at 260 °C). The SPME conditions were 15 min adsorption at 100 °C and 2 min desorption in split mode (30mL/min at 260 °C). The helium carrier gas was set at a constant pressure of 100 kPa. The chromatographic conditions included an initial oven temperature of 60 °C held for 2 min and a temperature ramp of 30 °C/min to 300 °C held for 15 min. The mass spectrometer was operated in the full scan EI+ (70eV) mode from 25–400 amu. The source and transfer line temperatures were set to 200 °C and 250 °C respectively. The detector multiplier was set to 500 V.
Table 3 represents the mass ions of the CAPs, ethanol and propyleneglycol. (5).

Table 3: Chromatograms of three major defence sprays.
Pepper spray standard preparation: The aerosol defence pepper spray chosen from the different standards was First Defense MK-3 spray (First Defense, Wyoming, USA) (Figure 3) from a police department in Ghent-Belgium. Pure pepper spray liquid (10 μL) was spiked onto a white cotton swab (15 × 15 cm2). This sample was put in a polyethylene bag and a nylon-11 bag. Another cotton swab without spiking was used as blank sample and preserved in the same conditions as above. A solution of 5% pepper spray liquid in methanol was prepared in a glass vial with screw cap.
Sample preparation: The clothing [100%] was confiscated in a polyethylene bag by the police force at the end of 2008. Two years later the whole packaging was placed in a nylon-11 bag in our laboratory.

Figure 3: Photograph of pepper spray canister investigated.
Head space conditions: GC septa were put on the several bags (clothing 100%; spiked cotton swab and blank cotton swab) with solvent-free aluminum tape and heated at 100 °C for 1 h in an oven. A 1 mL head space sample and an SPME adsorption sample (15 min) was taken and injected with a split of 30 mL/min at 260 °C (SPME for 2 min) (2).
Methanol solvent extraction conditions (1): Parts of the clothing were cut (back part [Control] as a blank, neck part front [N], left pocket front [L] and right pocket front [R]) with a Stanley knife and extracted with 50 mL methanol (a minimal volume to wet the fabrics [Figure 4]).
The extracts were manually shaken every 15 min for 24 h. The methanol was collected with the aid of a glass Pasteur pipette, in a glass jar. The liquids were evaporated with nitrogen to volumes of 100 μL respectively. These volumes were transferred to glass vials with screw caps and inserts.

Figure 4: Samples were collected from different parts of the jacket using methanol.
Results and Discussion
The case investigated involved a car chase in November 2008; driver X claimed to have been sprayed by driver Y with an aerosol defence pepper spray. The result of this action is driver X assaults driver Y. When the police force arrives, driver Y claims to have been sprayed. The police documented the symptoms of the assault on driver X. Driver Y only showed symptoms of beating. Only the protective cap of an aerosol defence pepper spray canister was found at the location. On interrogation the son of driver Y states that his father has an aerosol defence pepper spray and that because driver Y wasn't with his usual car, driver Y must have had the spray in his clothing. Therefore, the clothing of driver Y was confiscated from January 2009 for further analysis.
The following assumptions were made to clarify who was the victim or the attacker:
1. By analyzing the neck part [N] the possibility of a person being sprayed on could be determined.
2. By analyzing the right pocket part the possibility of a person having the spray in their possession could be determined.
This also assumes both parties were righthanded.
Our analytical method is capable of identifying the three major aerosol defence sprays (CN, CS and OC (the active components of CAPs); CN in head space mode, and CS and OC in solvent mode (Figure 5).
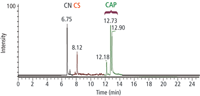
Figure 5: Chromatogram of three major aerosol defence sprays.
Head space extraction: By using mass specific ions 31 and 45, retention time comparison with pure liquids and NIST database identification, traces of ethanol and propylene glycol were detected on the clothing (the whole garment in a bag combination) (Figure 6). This indicates that the garment could have been in contact with a pepper spray.
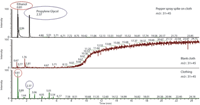
Figure 6: Traces of ethanol and propylenglycol.
Solvent extraction: The most common solvents used for extraction of the CAPs are methanol, acetone, methylene chloride and ethyl acetate. Table 3 summarizes the analytical results. By using mass specific ions: 137 (the most intense ion); 152, 195, 293 (the molecular ions of nordihydrocapsaicin); 305 (the molecular ion of capsaicin); and 307 (the molecular ion of dihydrocapsaicin), retention time comparison with pure liquid extracts and NIST database identification, traces of CAPs were only detected on the right pocket part of the clothing (Figure 7) (1) (4) (5). This indicates that the garment belonged to the attacker who was right-handed and that he was never sprayed on. There were no traces on the neck part,
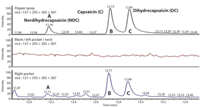
Figure 7: Traces obtained from pocket part of jacket.
Conclusions
CAPS can persist on clothing for a relatively long period of time if properly packaged and stored. Glass jars and polymer bags provide the best packaging for the collection and preservation of this type of evidence. Folding of the clothing and minimal air in the packaging are also important factors.
One must carefully select the parts of the clothing to be extracted. Context information is very important (defining victim and aggressor). As the fabrics must be cut up, permission must be obtained from the owner of the clothing. Methanol was found to recover all three compounds of interest in the clothing parts.
GC–MS is an excellent rapid technique for analyzing clothing containing different types of residues from pepper spray formulations. The detection of carriers or propellants by head space analysis (syringe or SPME) can provide additional evidence that a pepper spray has been used.
Gino Van Vaerenbergh has worked for 17 years as an expert in fire inverstigation by chemical analysis at the National Instiute of Criminalistics and Criminology in Belgium. He has a Masters degree in Industrial Engineering (Chemistry) and Environmental Technologies.
References
(1) K. Lewis and R.J. Lewis, J. Forensic Sci., 46 (2), 352–355 (2001).
(2) K.G. Furton et al., J. Chromatogr. Sci., 38, 297, (2000).
(3) O. Spicer andJ.R. Almirall, Elsevier Talanta,67(2), 377–382 (2005).
(4) P. Manirakiza, A. Covaci and P. Schepens, J. of AOAC Inter., 82(6), 1399–1405, (1999).
(5) L.K. Hin et al., MalaysianJ. Analytical Sci., 12(1), 210–216 (2008).
(6) Quadrex Analyses Guidebook: www.quadrexcorp/pdf.htm
(7) Shimadzu Pharmaceutical Analysis Guidebook: http://files.rushim.ru/books/chromatographia/analysis-guidebook-pharmaceutical.pdf;
Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.
Quantifying Microplastics in Meconium Samples Using Pyrolysis–GC-MS
March 26th 2025Using pyrolysis-gas chromatography and mass spectrometry, scientists from Fudan University and the Putuo District Center for Disease Control and Prevention detected and quantified microplastics in newborn stool samples.












