Analysis of Volatile Organic Compounds in the Environment Using TD-GC–MS
Exploring a new TD method capable of simultaneous analysis of compounds with a wide range of boiling points, high sensitivity, and high restore rates.
enzozo/stock.adobe.com

Air pollution is monitored by measuring the concentration of volatile organic compounds (VOCs) in a wide variety of environments. Multiple tubes are typically used to collect back samples or thermal desorption (TD) restore functionality is used, VOCs are trapped in a trap tube, the components in the sample gases that were split off before injection into the column are then trapped again. Consequently, the restore function ensures the sample can be re-measured even if a problem occurs during analysis. A newly developed thermal desorption (TD) method can help avoid the risk of wasting precious VOC samples, while enabling simultaneous analysis of compounds with a wide range of boiling points with sensitivity and high restore rates.
Measuring the concentration of volatile organic compounds (VOCs) in the air serves as a means of determining air pollution and is applied to monitor pollution in a wide variety of environments. However, because the method requires a long time to sample the atmosphere, multiple tubes are typically used to collect back samples, or thermal desorption (TD) restore functionality is used to reduce the risk of failing to analyze the VOCs.
The TD restore function thermally desorbs the sample gases trapped inside the sample tube and then restores the split sample gases in the tube before injection into the GC–MS system. Specifically, as shown in Figure 1, volatile components thermally desorbed from the sample tube are trapped in a trap tube (Figure 1, top). the trap tube is then heated to desorb the components and inject them into the column.
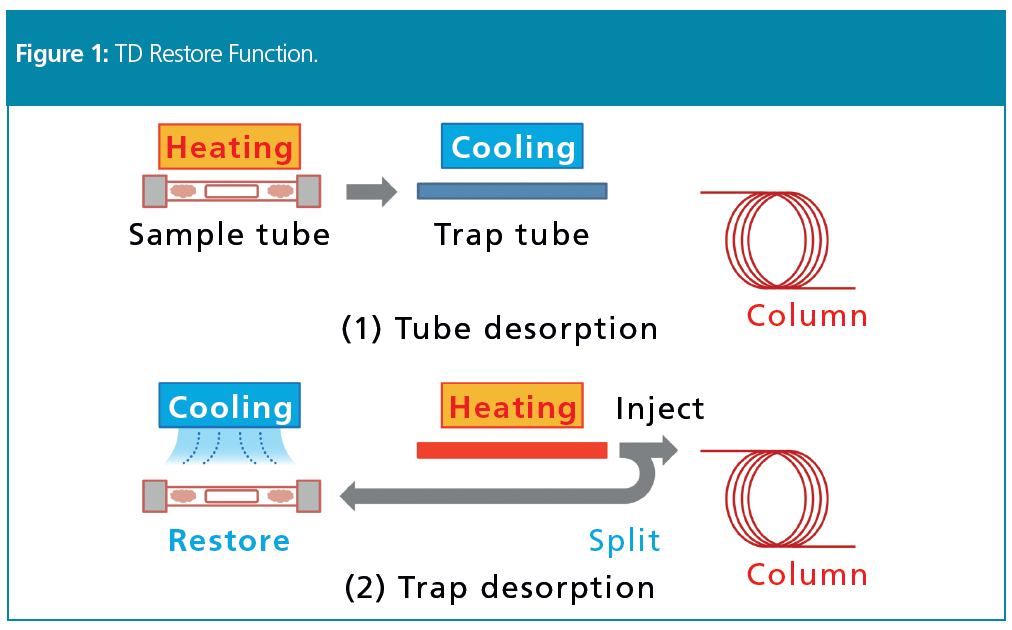
The components in the sample gases that were split off before injection into the column are then trapped again in the sample tube (Figure 1, bottom). Consequently, the restore function ensures that the sample gas can be re-measured easily, even if a problem occurs during analysis.
However, it is difficult to restore low boiling-point compounds while the sample tube is still hot immediately after desorption. This means that the sample tube must be cooled quickly before restoring low boiling‑point compounds and that the sample line temperature setting must be as low as possible.
On the other hand, if the sample line temperature is too low, it could increase the likelihood of carryover for high boiling‑point compounds. To address these concerns, the thermal desorber pretreatment unit moves the sample tube away from the heat source while blow‑cooling the sample during restoring, enabling faster cooling than previously possible. In addition, the possibility of analyzing compounds with a wide range of boiling points with high sensitivity and a high restore rate was investigated.
Method
Measurement Sample Gas Preparation: Components from one to four litres of air from the atmosphere (the sample gas) were trapped by an adsorbent in a sample tube. Carboxen (Merck) was used as the adsorbent. It was analyzed within 30 days (stored at 4 °C max.) after trapping the sample gas components. Toluene-d8 was added as an internal standard substance before analysis. Sample gas components trapped in the tube were dry‑purged with carrier gas before injection into the GC–MS system.
Analytical Conditions: A GCMS-QP2020 NX SQ was used as the GC–MS system. The thermal desorption (TD) method with a TD‑30R pretreatment unit was used for sample gas injection. Detailed analytical conditions are indicated in Table 1, that is, the final optimized TD-30R analytical conditions.
Analysis Results
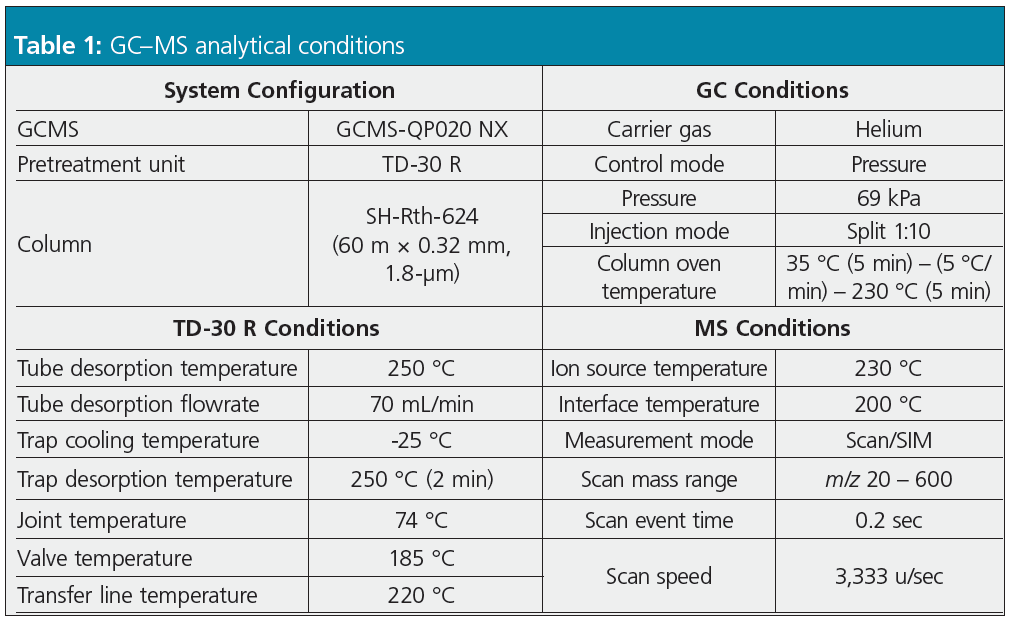
Study of Trap Tube Cooling Temperature: Due to the higher volatility of low boiling‑point compounds, they are more likely than high boiling-point compounds to remain untrapped if the trap tube is not sufficiently cooled, resulting in poor analytical sensitivity. The relationship between trap tube cooling temperature and sensitivity for analyzing low boiling-point compounds was considered at three trap tube cooling temperatures: 0, -20 and -25 °C. Chromatograms for the compounds with particularly low boiling points detected in the given sample (a) chloroethene and (b) ethyl chloride are shown as examples in Figure 2. Chloroethene has a very low boiling point of -13 °C, but it can be detected with high sensitivity by lowering the trap tube cooling temperature to -25 °C.
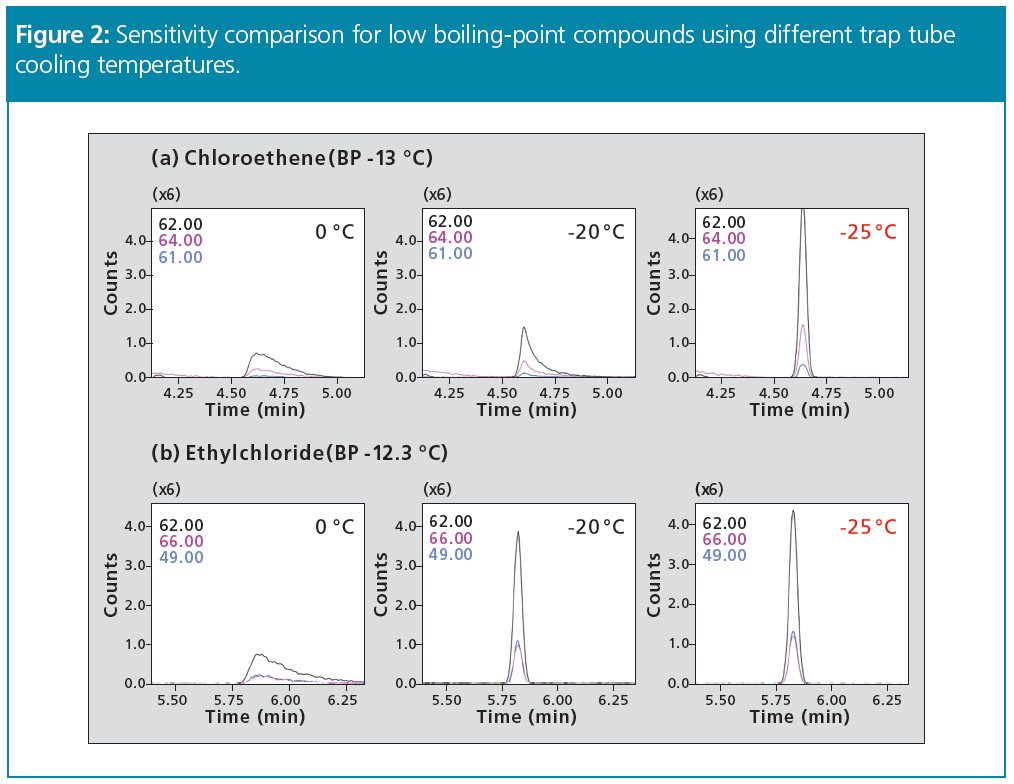
Study of Sample Tube Desorption Temperature: For thermal desorption, the sample tube and adsorbent can be reused as many times as desired by reconditioning the sample tube. However, if the sample tube thermal desorption process is insufficient, sample gas components can carry over from the previous measurement and affect the next measurement results. Consequently, the sample tube desorption temperature and flowrate have to be considered. Chromatograms for two compounds (a) 1,2,4-trichlorobenzene and (b) hexachloro‑1,3-butadiene that carried over from among all the compounds detected in the given sample gas are shown in Figure 3.
Before optimization, the sample tube desorption temperature was 250 °C (5 min) and the tube desorption flowrate was 30 mL/min. After optimization, the sample tube desorption temperature was 250 °C (10 min) and the tube desorption flowrate was 70 mL/min. Given the conditions before optimization, the carryover value for (a) 1,2,4-trichlorobenzene was 5.4%. In contrast, by specifying a longer thermal desorption time and a higher flowrate during desorption, as specified in the conditions after optimization, sufficient sample gas desorption occurred, and the carryover value decreased to 0.7%.
Reproducibility and Restore Rate of Restore Function: Optimal analytical conditions were determined from the studies described above. Reproducibility and restore rates for the restore function were then determined for those conditions. First, a standard sample gas (mixture containing 1 μg each of 40 substances) was added to six sample tubes to determine whether or not adequate measurement reproducibility was achieved based on initial analysis and restore results. These measurement results are shown in Table 2.
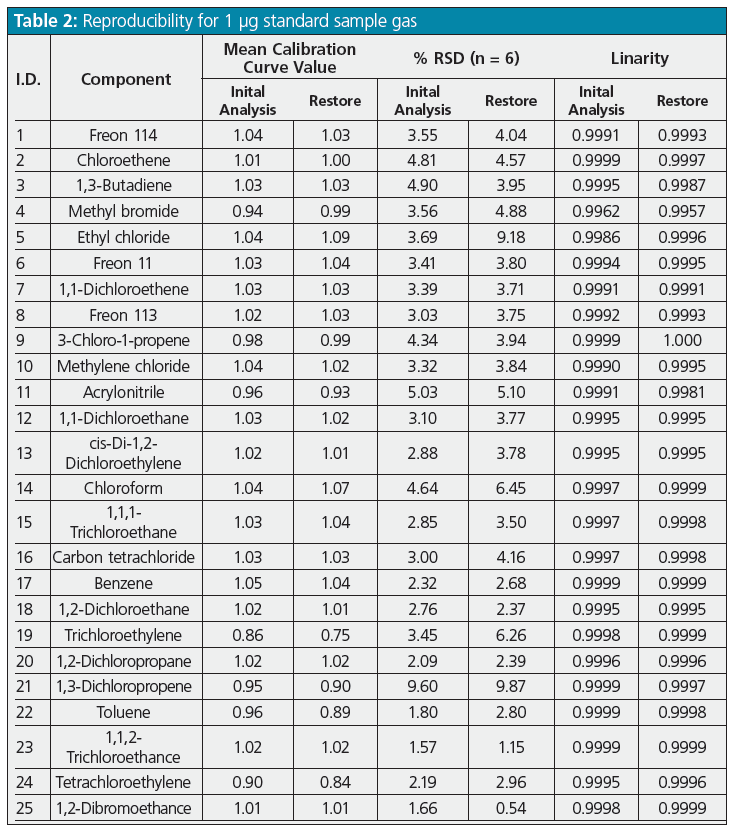
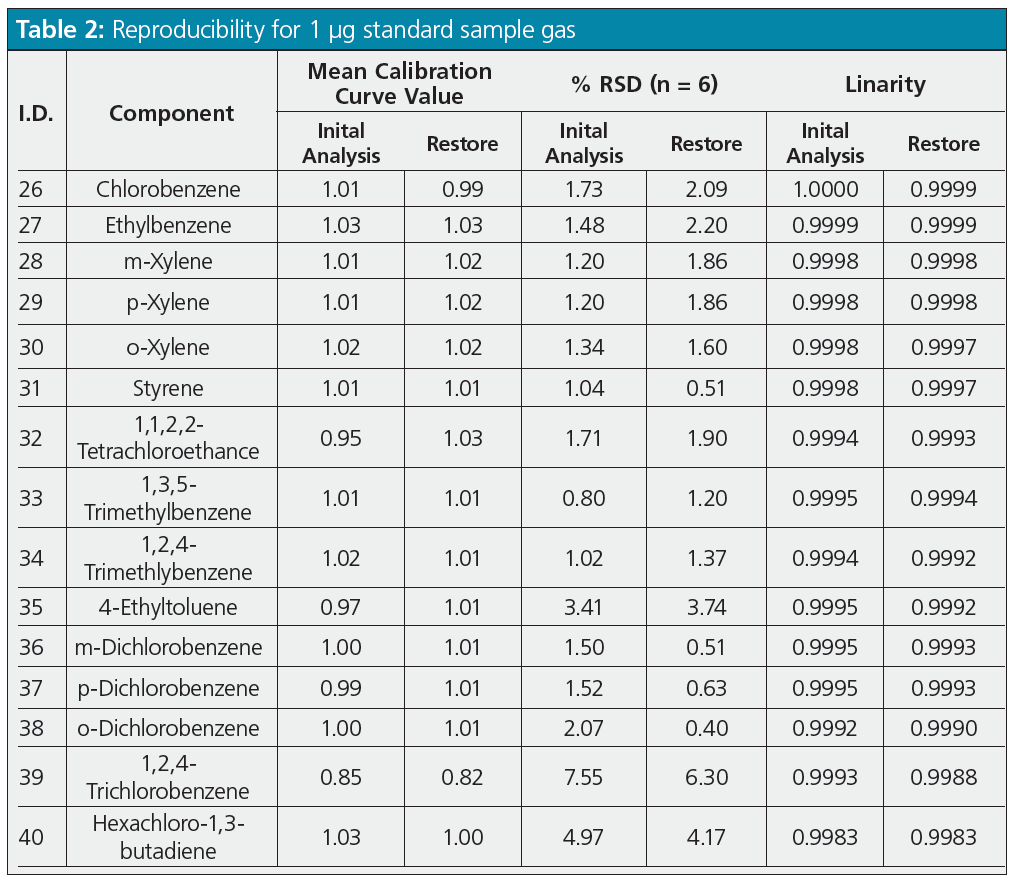
Mean values from measuring the six sample tubes were used as the standard sample quantitation values. Quantitation values were calculated from the calibration curve based on the peak area ratio for each concentration obtained by adding 1 to 50 μg of the standard sample. Table 2 shows the linearity of the calibration curve and the relative standard deviation (% RSD), which is within 10% for all substances, and the % RSD value was about the same for both the initial analysis and restoring for most substances, indicating good analytical reproducibility even when using the restore function. The difference between the % RSD value for the initial analysis and restore was large only for ethyl chloride (I.D. number 5 in Table 2). This large difference was presumably caused by the larger difference in recovery rates between ethyl chloride and toluene-d8, used as an internal standard substance during restoring, than for other substances.
Conclusion
The authors developed a TD method capable of simultaneous analysis of compounds with a wide range of boiling points, high sensitivity and high restore rates. With the optimized analytical conditions, VOCs were analyzed using restore with accuracy levels equivalent to initial analysis. This approach can help to avoid the risk of wasting precious VOC samples, but also to enable simultaneous analysis of compounds with a wide range of boiling points, while also achieving high sensitivity and high restore rates.
Waldemar Weber started his studies as a chemist in 2005 at the University of Münster, Germany. There, after graduating as a chemist, he achieved his Ph.D. in 2011 and completed a postdoc in 2013 at the Münster Electrochemical Energy Technology (‘MEET’) Research Center. From 2012 to 2013 he was employed as an Application Specialist at JAS in Moers, Germany. In November 2018 he joined Shimadzu Europa as Product Manager for GC–MS.
E-mail: shimadzu@shimadzu.eu
Website: www.shimadzu.eu

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.














