Environmental Analysis with Integrated Ion Chromatography, Titration, and Direct Measurement
Uncovering the benefits of a system that hyphenates ion chromatography, titration, and direct measurement, in environmental analysis of water samples.
dlux/stock.adobe.com

Environmental analysis generally involves more than one measurement technique to capture the full story behind a water sample. Determining the conductivity, pH, alkalinity, and ionic balance can take a significant amount of time for an analyst, especially managing different systems, software, and data management tools. This article describes the benefits of a system that hyphenates ion chromatography, titration, and direct measurement.
Water, the source and basis of all life, is the one thing that we all depend upon. It is essential for metabolism and is our most important foodstuff. Monitoring the water quality is vital to society and any technical solution to do so should be easy to use, reliable, and of course, both sensitive and highly accurate to deliver the best data. There is a serious need in water quality laboratories for cost‑effective and fast instruments and methods that can deal with the increasingly complex array of harmful substances, the increasing throughput of samples, and the decreasing detection limits set by various norms and standards.
Whether working in the field of environmental analysis, life sciences, or the food and beverage industry, analysts monitoring various water quality parameters know that a single technique cannot be used to measure everything necessary to form an in‑depth analysis of the sample. Measuring several variables such as conductivity, temperature, pH, hardness, anions, and cations on separate instruments is usually the norm. This piecemeal workflow requires significant time and manual labour, not only for the analyses, but also for reporting purposes.
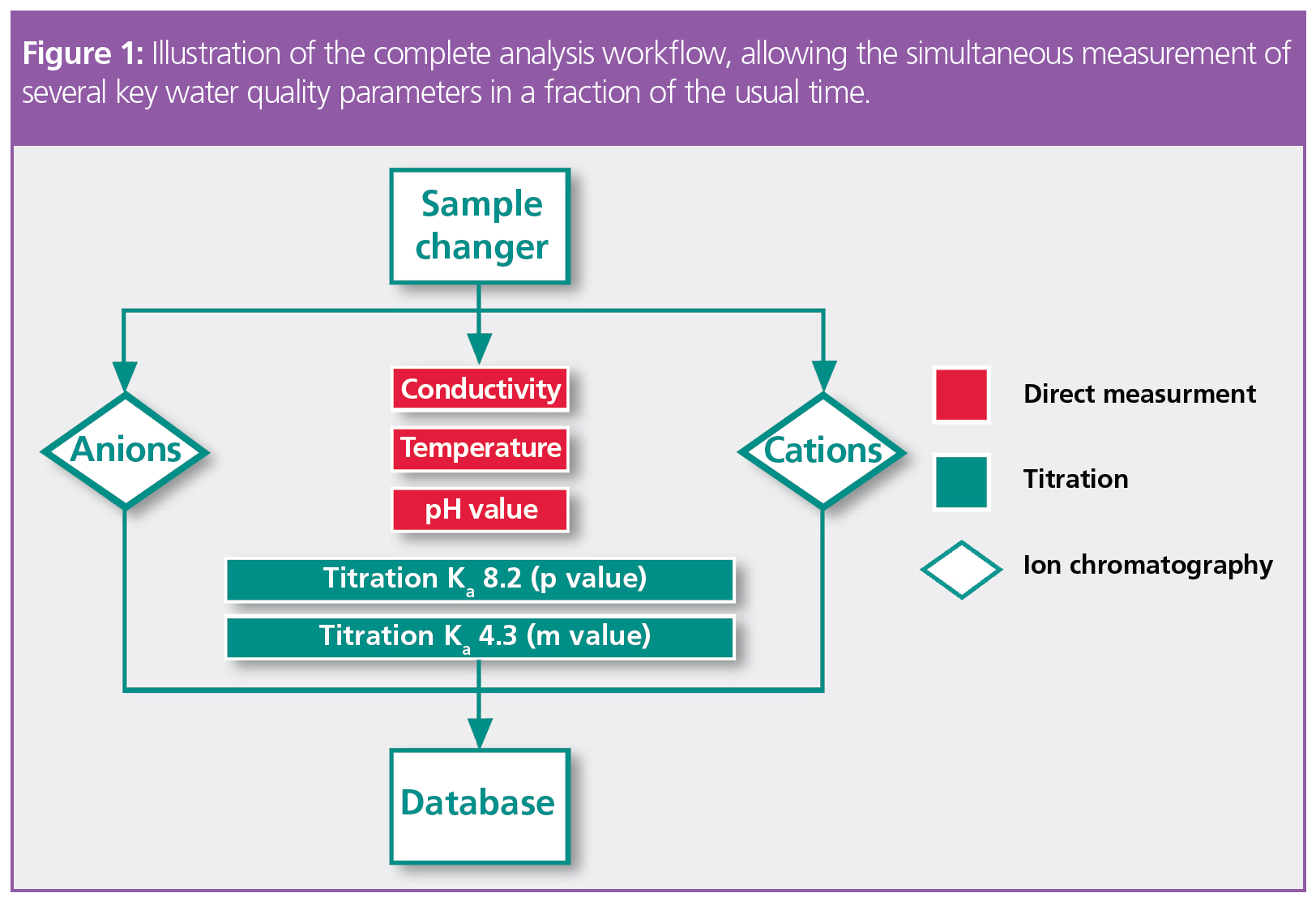
Combining measuring techniques such as titration, direct measurement, and ion chromatography (IC) in a single hyphenated system allows faster, more reliable, fully automated analysis of key water quality parameters. These include all the variables mentioned above, as well as the determination of the ion balance (an estimate of the analysis accuracy): uncomplicated comprehensive water analysis from one system.
IC, Titration, and Direct Measurement for the Analysis of Water
IC is one of the preferred technologies used for water analysis because it is ideal for the separation and quantification of ionic substances, even in complex matrices. While calibration with known standards is a must for this technique, the advent of smart software and automated sample preparation has made this task incredibly simple and results in excellent correlation values. IC is also mentioned in many international norms for water quality (for example, United States Environmental Protection Agency [EPA], ASTM International, Deutsches Institut für Normung [DIN], and so on), making it an excellent choice for laboratories working in this sector.
Titration is one of the most used methods for analysis in almost any industry for the measurement of hundreds of compounds. Titration is an absolute and robust chemical method—straightforward with no need for calibration, and is also stipulated in many standards, including ASTM and the International Organization for Standardization (ISO), for example. The sheer variety of measurement options, as well as the ability to use masking agents for interfering components, allows analysis of an array of substances with large concentration ranges. With titration, it is not only the ionic species that are measured, which broadens the amount of water quality variables that can be assessed beyond IC alone.
Direct measurement, which includes basic parameters such as temperature and conductivity, cannot be understated. These variables should always be monitored to obtain the most accurate results from the other techniques. Without this information, the error propagation from other measurements can become quite substantial.
Benefits of Integrated Analysis
The complete range of ions typically present in water can now be determined automatically with comprehensive, hyphenated water analysis instruments. The specific combination of titration, direct measurement, and IC in one system allows for thorough investigation of the water quality. Separately, these techniques require significant laboratory space and analyst time for sample preparation and measurement. Utilization of autosamplers and automated sample preparation steps can improve the workflow, however data must still be compiled from different sources.
The additional benefit of a hyphenated analysis system is that results from all three methods are collected in a single database and edited in a shared report. A common chromatographic analysis software is used to operate the system, making it even easier for adoption in the laboratory without the need for further user training. Data traceability, especially for laboratories with rigorous auditing requirements, is now possible for the entire workflow—from the chemical batch and separation column information to the results themselves.
Extended Measurement Possibilities
The following parameters and others can be automatically monitored in this comprehensive analytical system for general water analysis: pH value; temperature; conductivity; p and m value (alkalinity); hardness (calcium/magnesium); cations (for example, lithium, sodium, ammonium, calcium, and so on); anions (such as fluoride, chloride, bromide, nitrite, nitrate, phosphate, and so on); and ionic balance. Physical parameters such as turbidity and colour index can also be integrated at any time. With this combination of techniques, sum parameters including the conductivity as well as the ionic balance can be reported, adding another level of integrity to the results. For example, electrical conductivity provides valuable information regarding the salt content of a water sample. The total ionic balance of a water sample can assess the accuracy of the other techniques from which the individual values are generated. If this balance is significantly non‑zero (that is, above 10%), this would indicate an imbalance between total anions and cations represented in the measurements, and an investigation into the reasoning is required.
Experimental
IC Analysis: Analysis of the major anions and cations was performed on a 930 Compact IC Flex instrument (Metrohm) with conductivity detection using a Metrosep A Supp 17 - 150/4.0 separation column for anions (Metrohm) and a Metrosep C4 - 50/4.0 separation column for cations (Metrohm).Both the cation and the anion eluents were prepared from chemicals supplied by Sigma Aldrich. Ultrapure water (resistivity >18 MΩ·cm [25 °C], type I grade) was supplied by Elga. Anion suppressor regenerant solution was prepared from 96% sulfuric acid (Merck) and ultrapure water (Elga). All cation and anion standards for IC were made using the TraceCert range (Sigma Aldrich) and ultrapure water (Elga).
Direct Measurements: Both the direct conductivity measurements and the sample temperature were taken using a 856 Conductivity Module (Metrohm) with relevant electrodes. All pH measurements were performed with a digital electrode (dAquatrode) (Metrohm) combined with a temperature sensor (Pt1000) (Metrohm). The conductivity standard and pH buffers used in this study were sourced from Metrohm.
Titration Measurements: All potentiometric titrations were performed using an Omnis Advanced Titrator (Metrohm) and a Sample Robot (Metrohm). Hydrochloric acid (0.1 mol/L) titrant was bought directly from Merck, while the tris(hydroxymethyl)‑aminomethane (TRIS) used for the titer determination (>99.7%) came from Sigma Aldrich.
Analysis Software: For all measurements, MagIC Net 3.3 Professional chromatographic software (Metrohm) was used as a master to control the Omnis software (Metrohm), which operates in the background for other tasks.
Results and Discussion
Analysis of the anions and cations using IC takes approximately 26 min (Figure 2), although this duration can be optimized by adjusting several variables. During this time, the other parameters are automatically measured on the same sample, under the same conditions (Figure 1, Figure 3, Table 1).
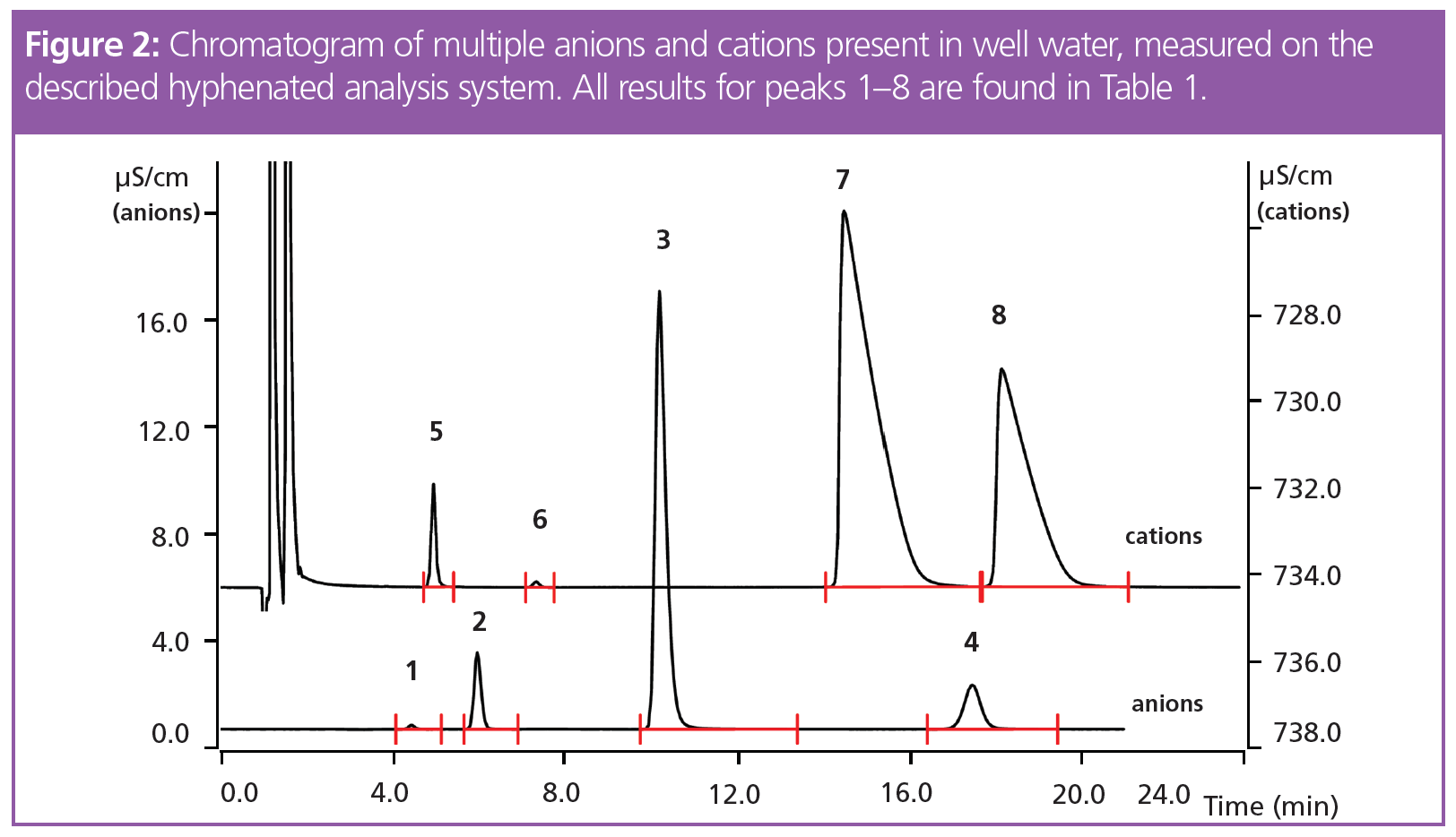
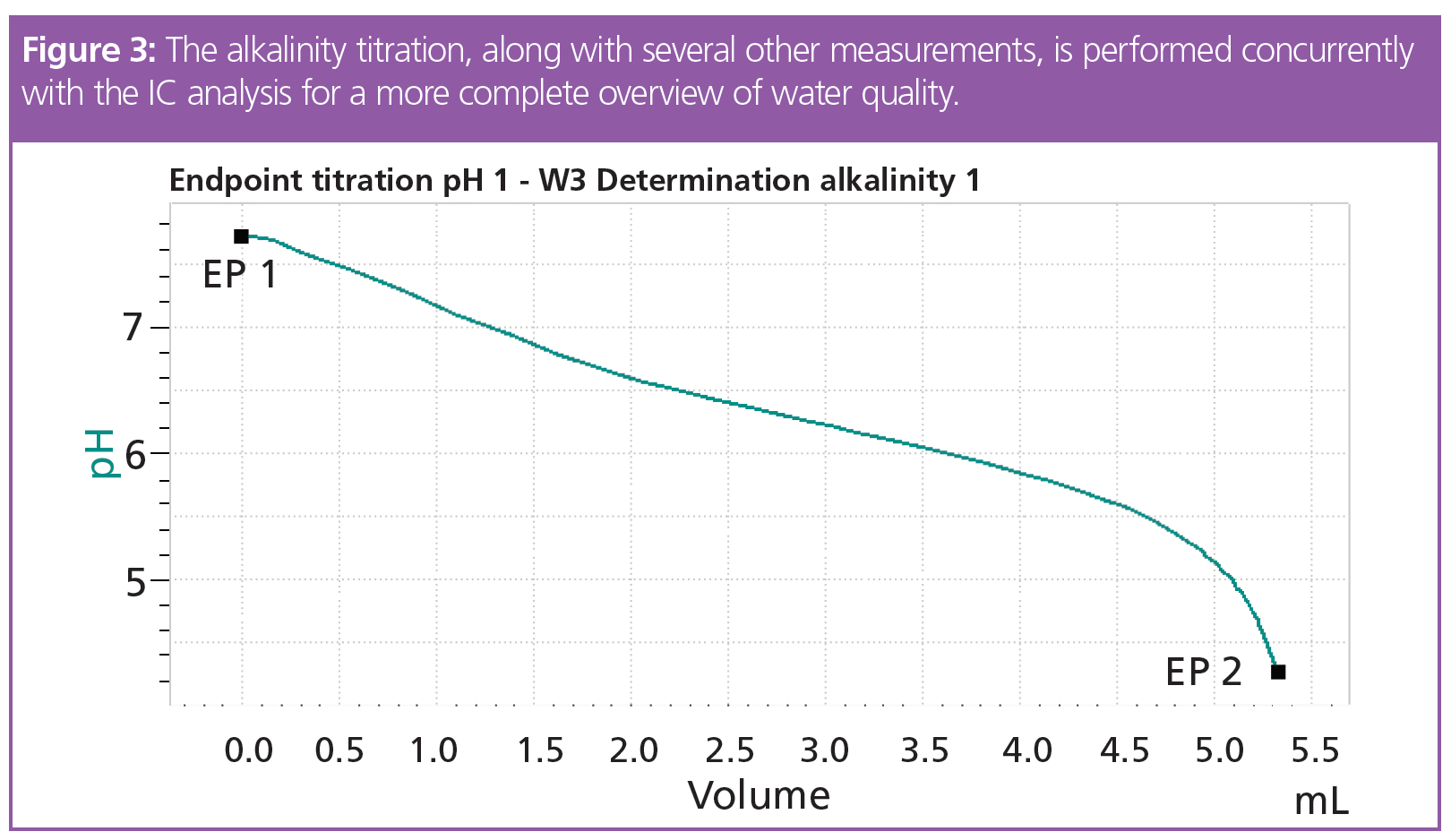
The results from the anion and cation determination (Table 1) lie within the Swiss regulation for drinking water (1) with good reproducibility. The s(rel) of the results is under 1.956% (n = 6) except for fluoride. Here, the high s(rel) is most probably caused by the small area counts of fluoride, where all integration variation has a significant effect on the final result. The absolute standard deviation is only 0.003 mg/L, which is completely acceptable.
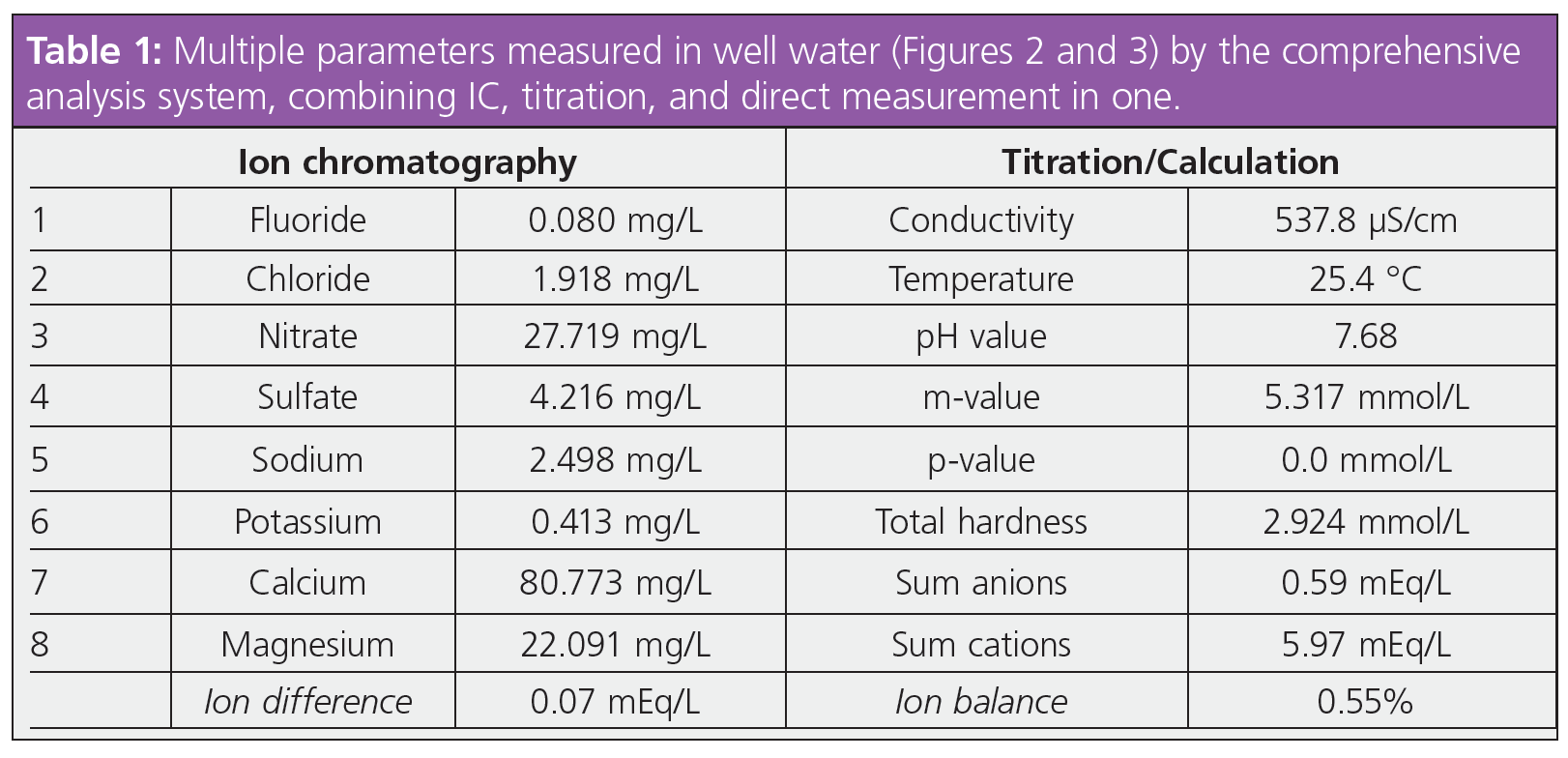
The value of 0.55% for the calculated ion balance in this example is well within expectations for a properly calibrated system (Table 1). This demonstrates the accuracy of the water analysis. An ideal value would be equal to zero, however, in reality this is almost never possible for a variety of reasons.
The integration of titration and IC in a single analytical system also allows users to check reported values for the same parameters (for example, calcium and magnesium) from different methods on the same sample at the same time. The ability to compare data from the same sample under such conditions gives analysts confidence in their results, without spending any unecessary additional time.
Analysts also have the choice of combining different possibilities such as the comprehensive analysis, only IC, only conductivity, or only titration of the sample, depending on their requirements.
Summary
Combining multiple analysis techniques in a single hyphenated system leads to reliable water quality results and more time to spend on other essential duties. Results are gathered in a common database and printed in a single report, reducing the need to compile data from several sources and software. Complete water quality analysis is made easier with the elimination of time‑consuming manual tasks such as sample preparation, dilution, calibration, and eluent preparation.
Performing these measurements and calculations on separate instruments takes hours longer than with an automated, integrated system.
Reference
- “Verordnung des EDI über Trinkwasser sowie Wasser in öffentlich zugänglichen Bädern und Duschanlagen” (Ordinance of the EDI on drinking water and water in publicly accessible bathrooms and shower facilities) (817.022.11, Stand vom 1. Mai 2018).
Alyson Lanciki is currently the Scientific Editor of Metrohm AG. In 2010, she received her Ph.D. in Analytical and Environmental Chemistry from South Dakota State University, USA. After a post‑doctoral position in France, Alyson joined the Metrohm family in 2013 as an ion chromatography application specialist in the Netherlands at Metrohm Applikon, where she eventually became the global marketing manager for the Metrohm Process Analytics brand of instruments.
Jennifer Lüber is currently the Product Specialist for Titration/TitrIC at Metrohm AG in Switzerland. She finished her apprenticeship as a Lab Technician at Metrohm AG in 2014. Afterwards, she was involved in projects related to column development, and worked in the laboratory for a short time at Metrohm UK. In 2015, Jennifer returned to Metrohm AG to join the competence center for titration, eventually taking responsibility for the TitrIC systems in 2018. Jennifer received her professional bachelor’s degree in business process management in 2019.
E-mail: info@metrohm.com
Website: www.metrohm.com

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.













