Analysis of Inorganic Anions by Capillary Electrophoresis
Capillary Electrophoresis is useful for analysing simple inorganic anions in forensic, clinical and pharmaceutical applications.
Capillary electrophoresis (CE) is well suited to the analysis of simple inorganic anions such as chloride, nitrate and sulphate. These analytes are water-soluble and highly mobile. Indirect UV detection is required as the analytes possess limited UV-absorbing properties. Electrolyte systems also require considerable optimization to accommodate the negative charge(s) on the anions. The methods are robust and rugged and have been applied to a variety of areas including pharmaceuticals, forensics and clinical applications.
What are the separation mechanisms used to analyse inorganic ions?
Separation of inorganic anions is achieved based on their electrophoretic mobilities; by their molecular weights and number of negative charges. Their mobilities are highly negative and oppose the normal electroendosmotic flow (EOF) direction. This situation would lead to very long analysis times and poor peak shapes and sensitivity. The EOF direction is therefore reversed to make it identical to the migration direction of the small anions, thereby reducing analysis times. A range of cationic surfactants have been employed at low mM concentrations, including hexamethonium [1,6-bis(trimethylammonium)hexane], tetraethylenepentaamine, 3 mM 1,3,5-benzenetricarboxylic acid and 15 mM TRIS in 20% (v/v) methanol at pH 8.4 in reference 1 and tetradecyltrimethylammonium bromide in reference 2. Figure 1 shows that the positively charged cationic surfactant binds onto the negatively charged capillary wall. A bi-layer of surfactant then forms which generates a positive charge on the capillary wall which reverses the EOF direction. A negative voltage is then used for analysis as both the EOF and the anions migrate in the same direction.
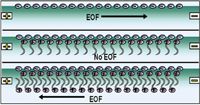
Figure 1: Reversal of EOF direction with cationic surfactant bilayer.
How is detection of inorganic anions achieved?
Simple anions such as chloride and sulphate have no chromophores so cannot be detected using UV detection. Detection of nonchromophoric metal ions (and those with limited chromophores) is therefore achieved by indirect UV detection. In this process a UV-absorbing species is added to the electrolyte which gives a high background signal to almost fullscale (FS) deflection. When the peak moves along the capillary it displaces the UV-absorbing species which leads to a decreased concentration and a dip in UV signal.
A high-mobility species is incorporated into the electrolyte to give the background UV signal. The background signal is reduced when the sample ion passes through the detector and the area of the peak generated is linearly related to the sample concentration. The migration speed of the UV-absorbing ion must closely match that of the ion being detected otherwise the analyte peak shape is severely distorted and poor sensitivity is obtained. Chromate3 and 1,2,4,5 benzene tetracarboxylic acid (pyromellitate)1 are popular choices as they give optimal peak shape and sensitivity for small anions such as chloride and sulphate. Species similar to benzoate are preferred for mediumsized anions such as citrate, phthalate and carbonate. Species similar to sorbate give optimal performance for larger inorganic anions such as alkyl sulphonates.
Figure 2 shows the separation of a range of common inorganic anions using pyromellitate as the UV absorber. Pyromellitate may be preferred to chromate, as chromate is potentially toxic and requires more handling care.
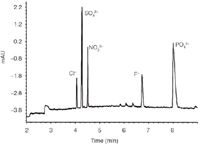
Figure 2: Separation of a range of inorganic anions by CE. 2.25 mM pyromellitic acid, 6.5 mM NaOH, 0.75 mM hexamethonium hydroxide, 1.6 mM triethanol-amine, pH 7; Negative polarity 465 V/cm, 9 mA; Capillary dimentions 56 à 64.5 cm i.d. 50 μm; Injection 4 sec at 50 mbar, Temp = 25 °C; Indirect detection signal: 350.60 nm, ref: 245.10 nm.
Electrolyte concentrations are kept low to generate low currents in the region of 5–10 μA. Alternatively zwitterionic buffers such as TRIS are used which do not carry current. Higher currents lead to an increase in temperature in the capillary which causes refractive index (RI)changes and excessively noisy baselines.
Chemistry kits are available from a number of capillary electrophoresis (CE) equipment vendors and consumable suppliers that are optimized for the analysis of inorganic anions by CE. The reagents supplied include capillary rinsing solutions and an optimized electrolyte solution. Operating conditions are recommended for parameters such as temperature, detection wavelengths and applied voltage. The kits also contain the capillary which has a length and diameter which give optimized performance using the recommended operating parameters. The kits may also contain test mixes of standard anions.
Typically, sample is injected into the capillary by dipping the capillary into the sample vial and applying a pressure which forces a few nanolitres of sample solution into the capillary. Alternatively it is possible to use voltage to selectively inject charged components from the sample solution into the capillary end. This is called electrokinetic injection and is achieved by dipping the injection end of the capillary into the sample solution. Small ions rapidly move into the capillary when the voltage is applied which results in preferential loading and improved sensitivity. For example, detection limits can be improved 10-fold for small inorganic anions compared with hydrodynamic injection.4 Typical sample loadings are achieved employing 30 second injections at –5 kV. Electrokinetic injection can be further optimized using sample additives such as octanesulphonate and have been used to monitor ppb levels of anions in nuclear power plant feed water. Good linearities and acceptable precisions can be achieved by electrokinetic injection, however the amounts loaded are highly dependent on the sample matrix which makes pressure injection more applicable to routine analysis.
What are the application areas?
CE has been used for a number of applications covering several industries and acceptable method validation has been reported with methods being routinely used.
Water analysis: Several samples of mineral water were analysed by CE and ion chromatography (IC) for chloride, sulphate, nitrate and fluoride.5 Table 1 shows the good agreement between the two techniques. Monitoring of the quality of the feedwater used in nuclear power plant is essential to avoid fouling of catalysts.4 Contamination occurs with ppb levels of anions and a CE method has been shown to be appropriate for monitoring a range of anions at that level.4
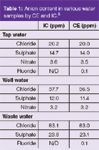
Table 1: Anion content in various water samples by CE and IC.5
Pharmaceutical analysis: CE has been widely used in the pharmaceutical analysis for anion determinations and the applications described would be appropriate in other industries such as agrochemicals. Basic drugs are often prepared as salts, with counter-ions, chloride or sulphate, manufactured to alter their physiochemical properties such as solubility. The counter-ion may typically constitute 5–30% of the weight of the output drug substance batch. Therefore it is essential that accurate and precise analytical methods are used to quantify counter-ion content.
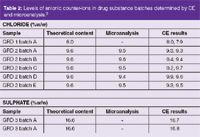
Table 2: Levels of anionic counter-ions in drug substance batches determined by CE and microanalysis.3
Table 2 shows the data obtained by CE for the analysis of the chloride counter-ion content of a basic drug.3 The CE data agrees well with the expected theoretical content and the values obtained by a manual titration method. The method was fully validated (Table 3) and used in routine analysis.

Table 3: Method validation data for a chloride assay using nitrate as an internal standard.3
Figure 3 shows the analysis of two different batches of a basic drug manufactured as a chloride salt. The analysis shows that one of the batches contained the expected level of chloride but also a range of other inorganic anionic contaminants.3 The CE method therefore offered a higher data quality than a simple test such as a titration.

Figure 3: Analysis of two batches of a chloride drug substance showing different levels of inorganic contaminants.3
Forensic analysis: CE has been used for both forensic analysis and profiling for 20 years. Tagliaro et al. have recently produced an excellent review article on the subject.6 CE is seen as a valuable alternative to IC particularly for the analysis of inorganic-based explosives because of its low running costs and its simplicity of use.
Early work by the FBI included profiling of explosive residues7 — the pattern of the inorganic anions in bomb residues is indicative of the ingredients used in bomb manufacture. CE that offers fast, selective and sensitive analysis of inorganic anions is compulsory for the identification of explosives in post-blast or environmental samples.
A CE method has been recently been reported for the simultaneous analysis of ten anions (chloride, nitrite, nitrate, thiosulphate, perchlorate, chlorate, thiocyanate, carbonate, sulphate and phosphate) which can be found in post-blast residues.8 The method also allows quatification of the azide anion which is often present in the composition of detonators. Formate is used as the internal standard and gives a 20 min total runtime. Intermediate precisions were 2.11% for normalized areas and 0.72% for normalized migration times. Limits of detection close to 0.5 ppm for all anions were obtained with the use of preconcentration techniques. Simple sample preparation was used, allowing the analysis of a large variety of matrices with the generic CE method. The method was applied to the analyses of real post-blast residues and detonator extracts. The CE results were compared with those obtained with the routine IC method and showed excellent correlation.
Clinical analysis: Profiling of inorganic anions can be used for diagnosing and monitoring critical conditions for metabolic disorders. On-site monitoring of urine anions has been reported using a microfluidic chip-capillary electrophoresis device.1 This allows simple bed-side rapid analysis of the highly variable anionic metabolites in urine samples, with samples injected neat or after simple dilution. A complete assay for four samples can be achieved within an hour for 15 anions commonly found in urines. Recoveries of more than 94% were obtained, with CE results agreeable with results obtained by a conventional ion chromatography (LC) method.1
Industrial sample analysis: Monitoring of trace impurities in electroplating baths are required to meet EU regulations for WEEE and RoHS and for quality control of electrodeposits. A CE method using a buffer containing 1.5 mM tetraethylenepentaamine, 3 mM 1,3,5-benzenetricarboxylic acid and 15 mM TRIS in 20% (v/v) methanol at pH 8.4 gave satisfactory repeatability with baseline separation for 14 organic and inorganic anions within 14 min.9 Good repeatability for migration time [0.32–0.57% Relative standard deviation (RSD)], satisfactory peak area and peak height (2.9–4.5 and 3–4.7% respectively), low detection limit (S/N = 2, 20–150 ppb) and wide working ranges (0.1–100 ppm) were obtained. The CE method was used to identify anion impurities which had been causing problems in aged tin baths.
A CE method has been reported for the simultaneous determination of inorganic anions, aliphatic and heterocyclic organic acids in various processed samples (commercial softwood and hardwood kraft lignin and two red wine samples of Pinot Noir grapes).2 The analytes were determined simultaneously in 10 min using an electrolyte containing 20 mM 2,3-pyrazine dicarboxylic acid, 65 mM tricine, 2 mM BaCl,2 0.5 mM cetyltrimethylammonium bromide and 2 M urea at pH 8.06. Linear plots for the analytes were obtained in the concentration range of 2–150 mg/L. RSDs of peak areas during a 3-day analysis period varied from 5.5% for glycolate to 9.5% for oxalate. RSDs of migration times varied between 0.4% and 1.1%. The detection limit (at S/N = 3) was 1 mg/L for all the analytes studied.
Why use CE for anion ion analysis?
Ion exchange chromatography (IEC) is widely used to determine inorganic anions. IEC columns are expensive and may have a short column life. IEC eluent purchase and disposal costs can be high. Specific IEC equipment is also required. In comparison, CE uses inexpensive fused-silica capillaries, low volumes of reagents and metal ion analysis is conducted on standard CE equipment, so no additional expense or training is required if a CE kit is already available. The set-up time for CE analysis is also very rapid — especially if kits are used, as there is no requirement with prepare any reagents. Compared with techniques such as gravimetric analysis and titration; CE offers advantages of improved selectivity and the highly automated system which allows unattended operation and automated results calculations.
CE is also able to tolerate injection of quite complex samples as the capillary can be aggressively cleaned between injections.
Conclusions
The simple and robust nature of the methods used for inorganic anion determination by CE has ensured that this has become a popular application. Standard separation conditions have been developed which give optimized performance. These standard conditions have allowed kits to be developed which greatly simplifies routine operation. Methods have been routinely employed in a number of application areas including forensic, clinical and pharmaceutical determinations.
Kevin Altria is an associate director in the pharmaceutical development department at GlaxoSmithKline. He is editor of CE Currents in LCGC Asia Pacific. Direct correspondence about this column to LCGC Europe editor Alasdair Matheson, Advanstar Communications, Bridgegate Pavilion 4A, Chester Business Park, Wrexham Road, Chester CH4 9QH, UK, or e-mail at amatheson@advanstar.com
References
1. H. Sun, K.M. Lau, Y.S. Fung, Electrophoresis, 31, 3044–3052 (2010).
2. S. Rovio, A. Kalliola, H. Siren and T. Tamminen, J. Chromatogr. A, 1217, 1407–1413 (2010).
3. K.D. Altria, M.M. Rogan and D.M. Goodall, Chromatographia, 38, 637–642 (1994).
4. P.E. Jackson and P.R. Haddad, J. Chromatogr., 640, 481 (1993).
5. J.P. Romano and J. Krol, J. Chromatogr., 640, 403, (1993).
6. F. Tagliaro, J. Pascali, A. Fanigliulo and F. Bortolotti, Electrophoresis, 31, 1–9 (2010).
7. K.A. Hargadon and B.R. McCord, J. Chromatogr., 602, 241 (1992).
8. C. Sarazin et al., J. Chromatogr. A, 1217, 6971–6978 (2010).
9. H. Sun, K.M. Lau and Y.S. Fung, J. Chromatogr. A, 1217, 3244–3250 (2010).


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)