Determination of 25-mono-hydroxy-vitamin D2 and D3 in Human Plasma Using a Symbiosis Pico System: ACN Free On-line SPE-LC–MS–MS
Spark Holland Application Note
Introduction
The method developed runs on a fully automated platform integrating solid-phase extraction (SPE) and LC (Symbiosis Pico) without any manual pretreatment step(s). Automatic pretreatment results in high throughput, low CVs and high precision. Sample preparation has been simplified to just one operator step before the system takes over the automated analysis of the samples.
Instrumentation
A Spark Holland Symbiosis PICO system with a Mistral column oven and an Applied Biosystems/MDS Analytical Technologies API 4000 LC–MS–MS system are used for this application. The API 4000 is equipped with a Heated Nebulizer (APCI) probe.
Symbiosis Pico is Spark Holland's unique (u)HPLC solution with integrated on-line SPE-LC–MS automation. The system offers large flexibility in processing different types of samples selecting one of the three fully automated operational modes LC; XLC (extraction liquid chromatography) and mXLC (multidimensional on-line SPE)
Experimental
Sample preparation consists of diluting a human sample with a special protein disrupting buffer, followed by mixing. Samples are then placed in the Symbiosis autosampler. 100 μL of sample is injected on a Spark HySphere C8EC-SE sorbent SPE cartridge for on-line sample clean-up and concentration.
The mobile phase consists of formic acid, water and methanol with separation on a Merck KGaA Chromolith SpeedROD RP-18e (endcapped), 50 × 4.6 mm HPLC column. An example chromatogram of the separation achieved is shown in Figure 1.
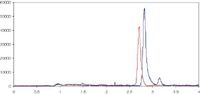
Figure 1: Chromatogram representing 100 ng/mL 25-mono-hydroxy-vitamin D3 (red) and 25-mono-hydroxy-vitamin D2 (Blue) spiked in human plasma.
Results
A calibration curve was created by injecting a full set of calibration standards ranging from 5 to 500 ng/mL spiked human plasma. The calibration curve is calculated with a R2 of 0.999 with a 1/X weighting for both compounds.

Table 1: Accuracy and precision of the QC samples.
Conclusion
This method was evaluated using spiked human sodium heparin plasma samples. The developed on-line SPE-MS–MS method has an absolute recovery of more then 90%, and calibration curve with a linear range from 5–500 ng/mL (R = 0.999 for both compounds). The developed method has an accuracy ranging from 89–117% and precision of 0.1–7.9 %CV. The estimated quantification limits for both analytes are more than sufficient to allow the analytical method to be used for therapeutic drug monitoring. When injecting 100 μL, containing 50 μL of plasma, a detection limit of 5 ng/mL is achieved.
Martin Sibum, Spark Holland bv, Emmen, The Netherlands.
References
1. Spark application note 0053-080-01.
2. Applied Biosystem Vitamin D i-method (User Contributed i-method by Spark).
For more information please send an e-mail to: Applications@sparkholland.com

Spark Holland bv
P. de Keyserstraat 8, PO box 3887800 AJ Emmen, The Netherlands
E-mail: Applications@sparkholland.com Website: www.sparkholland.com

Fundamentals of Benchtop GC–MS Data Analysis and Terminology
April 5th 2025In this installment, we will review the fundamental terminology and data analysis principles in benchtop GC–MS. We will compare the three modes of analysis—full scan, extracted ion chromatograms, and selected ion monitoring—and see how each is used for quantitative and quantitative analysis.
Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
This information is supplementary to the article “Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing”, which was published in the May 2025 issue of Current Trends in Mass Spectrometry.