Analysis of the Carotenoid Composition and Stability in Various Overripe Fruits by Comprehensive Two-Dimensional Liquid Chromatography
LCGC Europe
Carotenoids are a class of natural pigments, widely distributed in vegetables and fruits. A comprehensive LC×LC method, based on the use of a cyano and an octodecylsilica column, placed in the first and second dimension, respectively, was applied to evaluate carotenoid composition and stability in selected overripe fruits representing the waste generated by a local food market.
Francesco Cacciola1, Daniele Giuffrida1, Margita Utczas2, Domenica Mangraviti3, Marco Beccaria2, Paola Donato1, Ivana Bonaccorsi3, Paola Dugo2,3, and Luigi Mondello2,3, 1Dipartimento di “Scienze biomediche, odontoiatriche e delle immagini morfologiche e funzionali”, University of Messina, Messina, Italy, 2Chromaleont s.r.l., c/o University of Messina, Messina, Italy, 3Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, University of Messina, Messina, Italy.
Carotenoids are a class of natural pigments, widely distributed in vegetables and fruits. A comprehensive two-dimensional liquid chromatography (LC×LC) method, based on the use of a cyano and an octodecylsilica column, placed in the first and second dimension, respectively, was applied to evaluate carotenoid composition and stability in selected overripe fruits representing the waste generated by a local food market. This research also evaluates if post-climacteric biochemical changes are linked to carotenoid degradation in the investigated fruits. A total of 22 compounds was separated into seven different chemical classes in the two-dimensional space, and identified by photodiode array (PDA) and mass spectrometry (MS) detection. The results prove that the waste generated by the large distribution of food still represent an important source of bioactives that could be used for other purposes.
Carotenoids are a class of natural pigments, widely distributed in vegetables and fruits, and often responsible for the yellow reddish colour of many foods. Apart from their colourant and biological properties, several studies have demonstrated that carotenoids belong to a nutritionally important family of phytochemicals (1,2). Carotenoids are usually classified into two main classes: hydrocarbon carotenoids, generally known as carotenes (β-carotene, lycopene), and oxygenated carotenoids, known as xanthophylls (β-cryptoxanthin, lutein, violaxanthin) (3,4). Mono or dihydroxylated carotenoids often occur in an esterified form that is more stable than the free carotenoids. Moreover, esterification greatly increases during the fruits ripening process (5).
The wastes generated by the large distribution of food still represent an important source of bioactives, which could be diverted towards further uses, either in the animal feed production, or to the recovery of purified molecules for nutraceutical purposes. In the current study the carotenoid composition and stability in three overripe fruits, namely hybrid persimmon-apple, banana (pulp and peel), and nectarine was evaluated for the first time, thus also evaluating whether post-climacteric biochemical changes are linked to carotenoid degradation in the investigated fruits.
Comprehensive two-dimensional liquid chromatography (LC×LC) is a technique based on the combination of two independent separation steps with orthogonal selectivities. In LC×LC, a primary column is connected to one or more secondary columns by means of a switching valve as an interface. The function of the latter is to isolate continuous fractions of the first dimension column (1D) effluent and then release them onto the second dimension (2D) column; in this way the entire sample is analyzed in both dimensions, and very high values of peak capacities are obtained (6–10).
In the present research, a normal-phase LC × reversedâphase LC application has been developed, consisting of a microâbore 250 × 1.0 mm, 5-μm cyano column for the 1D separation, interfaced to a 2D C18 column packed with fused-core particles (30 × 4.6 mm, 2.7-μm), coupled with both photodiode array (PDA) and mass spectrometry (MS) detectors for the carotenoids evaluation in the previously mentioned fruits matrices.
Experimental
Chemicals: All the reagents and solvents used were of analytical- or HPLC-grade and were purchased from SigmaâAldrich. Carotenoids standards, namely β-carotene, lycopene, β-cryptoxanthin, lutein, and zeaxanthin, were purchased from Extrasynthese.
Sample and Sample Preparation: Overripe nectarine, hybrid persimmon-apple, and banana samples were purchased in a market place. For the extraction of intact carotenoids, 1 g of each lyophilized sample was treated with 4 mL of methanol, and then shaken with a magnetic stirrer for 15 min. An equal volume of hexane (Hex) (4 mL) was added to the mixture and shaken for another 15 min, as before. Subsequently, 3 mL of water was added to the mixture and shaken again. The mixture was centrifuged at 3000 × g for 15 min, and the organic layer was recovered in a volumetric flask and dried under vacuum. The dry residue was dissolved in methanol/methyl tert-butyl ether (MTBE) (1:1, v/v) mixture and then filtered through a 0.45 μm Acrodisc nylon membrane filter (Pall Life Sciences) prior to LC analysis.
LC×LC Instrument: LC×LC analyses were performed on a Prominence LC-20A (Shimadzu), consisting of a CBM-20A controller, four LC-20AD dual-plunger parallel-flow pumps, a DGU-20 A5 degasser, an SPD-M20A photo diode array detector (2.5 μL detector flow cell volume), a CTO-20AC column oven, and a SIL-20A autosampler (all Shimadzu). The two dimensions were connected by means of a 10-port two-position switching valve equipped with a micro-electric actuator (Sigma-Aldrich/Supelco) placed inside the column oven and equipped with two 0.254 mm i.d. stainless steel sample loops of identical volume (10 μL). Both dimensions and the switching valve were controlled by LCMSsolution software (Version 3.60.361, Shimadzu). The LC×LC data were visualized and elaborated into two and three dimensions using Chromsquare ver. 2.0 RC2 software (Chromaleont). The LC×LC system was coupled to an LCMSâ2010 mass spectrometer through an atmospheric pressure chemical ionization (APCI) source (Shimadzu).
LC×LC–PDA-MS Conditions:1D: 1D analyses were performed on an Ascentis ES Cyano column (250 × 1.0 mm, 5-µm SigmaâAldrich/Supelco).
1D Mobile Phase: (A) Hex; (B) Hex/butyl-acetate/acetone (80:15:5, v/v/v). Gradient: 0.01 min, 0% B; 34 min, 0% B; 50 min, 50% B; 60 min, 100% B; 180 min, 100%B. Flow rate: 10 μL/min adjusted by means of a flow splitter, (split ratio 1:20). Column oven: 40 °C. Injection volume: 5 µL.
2D: 2D analyses were performed on an Ascentis Express C18 column (50 × 4.6 mm, 2.7-μm Sigma-Aldrich/Supelco).
2D Mobile Phase: (A) H2O/acetonitrile (10:90, v/v); (B) (isopropanol) IPA. Gradient: 0.01 min, 20% B; 0.05 min, to 70% B; 0.63 min, 80% B; 0.75 min, to 90% B; 0.76 min, 20% B. Flow rate: 3 mL/min (splitted to 0.75 ml/min).
PDA Detection: 250–550 nm (sampling rate, 12.5 Hz; time constant, 0.08 s; cell temperature 40 °C).
MS Detection (APCI-Positive and Negative Ionization Modes): m/z range: 450–1100 amu; nebulizing gas (N2)
flow: 2.0 mL/min; detector voltage: 2.0 kV; interface temperature: 450 °C; CDL temperature: 300 °C; heat block temperature: 300 °C; event time: 0.15 s; scan speed: 6000 amu/s; CDL temperature: 300 °C; heat block temperature: 300°C.
Results and Discussion
The aim of this study was to evaluate the carotenoid composition and stability in three overripe fruits, namely hybrid persimmonâapple, banana (pulp and peel), and nectarine for the first time by the use of an LC×LC method, consisting of normal phase and reversed phase chromatography in the 1D and in the 2D, respectively. The two dimensions were both optimized independently, then combined and tuned together.
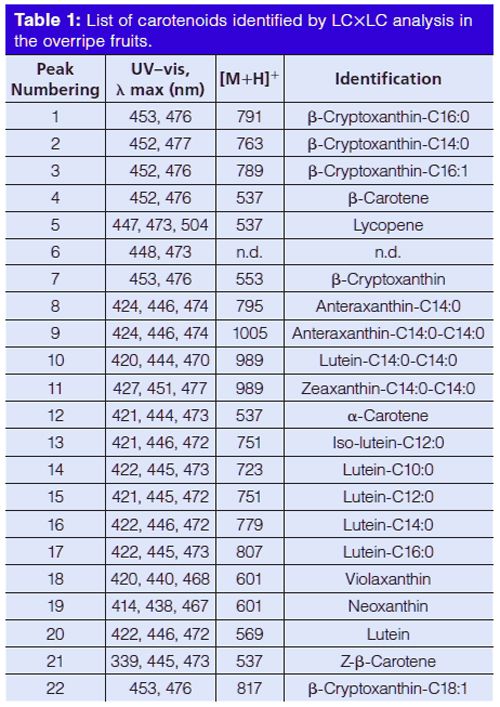
A micro-bore cyano column was chosen for the 1D separations and it was operated with a step-wise gradient, starting from 100% Hex (A, 0.01 min) reaching 100% (B) Hex/butyl-acetate, acetone (80:15:5, v/v/v) in 60 min. The mobile phase flow rate was set to 10 µL/min, to give the best results in terms of peak overlap and sampling, performing a carotenoid separation into classes of increasing polarity. The experiments were performed on an LC×LC instrument configured with an electrically actuated 10-port two-position switching valve, allowing automated fraction collection/reinjection of the two 10 µL loops. Aliquots eluted of the 1D were sampled in one of the sampling loops and transferred into the 2D via the 10-port valve, while the second sampling loop is filled. So, the whole effluent from the 1D column was transferred on-line to the 2D column, an octadecylsilica column every 60 s, and resolved using a gradient elution program, starting with a low concentration of the stronger solvent (IPA), 20% at mobile phase flow of 3.0 mL/min. Figure 1(a–d) shows the LC×LC chromatograms of the carotenoid extracts in (a) overripe hybrid persimmon-apple, (b) banana pulp and (c) peel, and (d) nectarine at 450 nm, with the location of the different identified carotenoid classes in the 2D space. Figure 2(a–d) shows the normal-phase LC × reversed-phase LC contour plots, indicating the peak numbering of the samples investigated (a: hybrid persimmonâapple; b: banana pulp; c: banana peel; d: nectarine). Table 1 reports the relative peak assignment for the spots numbering shown in Figure 2.
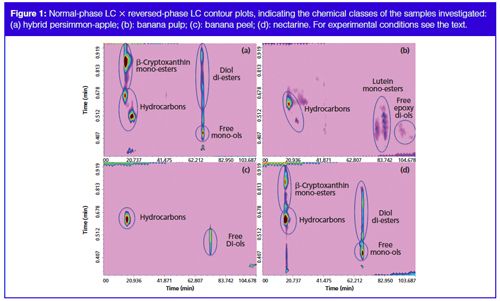
Chromatography on the cyano stationary phase allowed a good separation of the carotenoids in seven groups of different polarity in the first dimension, as can be seen from the circles in Figure 1, namely hydrocarbons, mono-ol-esters, di-olâdiâesters, di-olâmonoâesters, free-mono-ols, free-di-ols, and freeâepoxyâdiâols. On the other hand, the secondary C18 column allowed the separation of carotenoids within each class, according to their increasing hydrophobicity and decreasing polarity (for components of the same class, the elution order increases with the number of carbon atoms of the fatty acid chain).
Identification of the separated compounds was achieved by means of both PDA and MS detection (through APCI ionization). The latter represents a powerful analysis tool for unknown molecules; particularly in the case of carotenoids, operation of the interface under both positive and negative mode offers the double advantage of improved sensitivity and identification power. MS spectra obtained under negative ionization mode are in fact dominated by the presence of very intense pseudoâmolecular ions [M.]-, which makes identification of low-abundant components easier; on the other hand, abundant fragmentation is generally observed under positive APCI ionization, especially for carotenoid esters, whose fragment ions can help in structure elucidation. It must also be stressed that a better front-end LC separation is highly beneficial before MS analysis because clearer spectra are obtained.
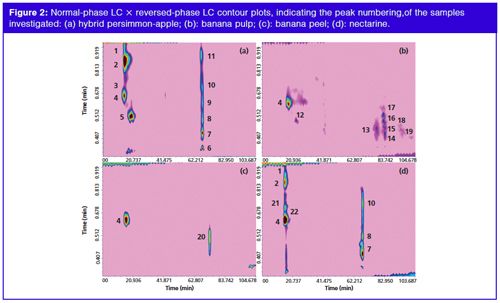
The combined use of PDA and MS data allowed 22 carotenoids contained in the samples to be distinguished, either in their free or in the esterified form (Table 1); it is noteworthy that the complementary information attained allowed compounds showing similar (or nearly identical) UV-absorption properties, arising from the same chromophore groups, to be discriminated between. An example is represented by the two monoâolâesters labelled as 2 and 1 in Table 1, namely β-cryptoxanthin-C14:0 and its superior homologue β-cryptoxanthin-C16:0. The absorption spectra of these two molecules in fact overlap, while the m/z pseudo-molecular ions are easy to distinguish one from the other. The studied fruits are representative of tropical and temperate zone fruits and are considered as climacteric fruits. In fact these fruits can be ripened after harvest; thus the present study also aims to evaluate whether postâclimacteric biochemical changes are linked to carotenoid degradation in the investigated fruits. Although some reports are available in the literature on the carotenoid composition of fresh apples (11) and persimmon (12), the carotenoid profile of their hybrid fruit had never been previously investigated. From the evaluation of the carotenoid profile shown in Figure 2(a) for the overripe kakimela, it can be concluded that both the hydrocarbons, β-carotene and lycopene, and the various identified xanthophyll mono- and di-esters were stable in the overripe sample; moreover, the carotenoids pattern in the hybrid persimmon-apple was more similar to the reported carotenoid profile of native kaki fruit then to the reported carotenoid profile of native apple fruits, thus indicating that prevailing metabolic pathways may occur in the carotenoid production of the investigated fruit. In agreement with the report by Lokesh et al. (13), β-carotene, lutein, and α-carotene were the main carotenoid detected in banana as shown in Figure 2(b) and (c); moreover, it can be observed here that free lutein was only detected in the pulp and that a series of lutein esters were only detected in the peel. It can also be observed that those carotenoids were stable in the overripe banana fruit. The stability of the main carotenoid in nectarine (14), β-carotene, in overripe nectarine was observed in this study together with other minor - here detected for the first time - carotenoid in this fruit, such as cis β-carotene and the xanthophylls esters.
Conclusions
The applied LC×LC methodology has enabled the identification of different carotenoids, including various esters, in selected overripe fruits and its application in the analysis of other complex carotenoid matrices could be a future objective of research. The results showed that no post-climacteric carotenoid losses occurred and that provitamin A carotenoids and lutein were indeed stable in the overripe stage of the studied fruits. Thus, those matrices still represent an important source of bioactives, which could be diverted towards further uses, either in animal feed production, or to the recovery of purified molecules for nutraceutical purposes.
Acknowledgements
The authors wish to thank the “Italian Ministry for the University and Research (MIUR)” within the National Operative Project PON04a2_F “BE&SAVE” “Technologies and operational models for the sustainable food chain management through the valorization of the Biological waste for Energy use and the reduction of the Scraps generated by the Alimentary distribution and consumers, treatment for the Valorisation of the Edible part of the urban solid waste and experimental development”.
We are pleased to thank the “University of Messina” within the “Research and Mobility” Project.
References
- G. Britton, S. Liaaen-Jensen, and H. Pfander, Carotenoids (Volume 5: Nutrition and Health. Birkhauser, Basel, Switzerland, 2009).
- N.I. Krinsky and E.J. Johnson, Molec. Aspect. Med. 26, 459–516 (2005).
- G. Britton, S. Liaaen-Jensen, and H. Pfander, Carotenoids Handbook (Birkhauser Verlag, Basel, Switzerland, 2004).
- M. Herrero, F. Cacciola, P. Donato, D. Giuffrida, G. Dugo, P. Dugo, and L. Mondello, J. Chromatogr. A 1188, 208–215 (2008).
- D. Hornero-Méndez, R. Gómez-Ladrón De Guevara, and M.I. Mínguez-Mosquera, J. Agric. Food Chem. 48, 3857–3864 (2000).
- P. Dugo, T. Kumm, F. Cacciola, G. Dugo, and L. Mondello, J. Liq. Chromatogr. Related Technol. 31, 1758–1807 (2008).
- P. Dugo, M. Herrero, D. Giuffrida, T. Kumm, G. Dugo, and L. Mondello, J. Agric. Food Chem. 56, 3478–3485 (2008).
- L. Mondello, P. Donato, F. Cacciola, C. Fanali, and P. Dugo, J. Sep. Sci. 33, 1454–1461 (2010).
- F. Cacciola, P. Donato, D. Giuffrida, G. Torre, P. Dugo, and L. Mondello, J. Chromatogr. A 1255, 244–251 (2012).
- P. Dugo, N. Fawzy, F. Cichello, P. Donato, and L. Mondello, J. Chromatogr. A 1278, 46–53 (2012).
- R. Delgado-Pelayo, L. Gallardo-Guerrero, and D. Hornero-Mendez, Food Res. Int. 65, 272–281 (2014).
- Y. Hitaka, A. Nakano, K. Tsukigawa, H. Manabe, H. Nakamura, D. Sakano, J. Kinjo, T. Nohara, and H. Maeda, Chem. & Pharm. Bull. 61, 666–669 (2013).
- V. Lokesh, P. Divya, B. Puthusseri, G. Manjunatha, and B. Neelwarne, LWT - Food Sci. Tech. 55, 59–66 (2014).
- D. Bohoyo-Gil, D. Dominguez-Valhondo, J.J. Garcia-Parra, and D. Gonzales-Gomez, Europ. Food Res. Tech. 235, 1055–1062 (2012).
Francesco Cacciola is an assistant professor of food chemistry at the University of Messina, Italy. His research interests include the characterization of complex food samples by conventional and innovative LC and MS techniques.
Daniele Giuffrida is an associate professor of food chemistry at the University of Messina, Italy. His research activity is mainly focused on the investigation of bioactive molecules in natural products and foodstuffs by means of advanced and multidimensional chromatographic techniques.
Margita Utczas is a GC–MS specialist at Chromaleont s.r.l., c/o University of Messina, Italy. Her main research skills are the development of supercritical technologies for both industrial and pharmaceutical applications.
Domenica Mangraviti is a PhD student in chemical sciences at the University of Messina, Italy. Her research is mainly focused on the development of comprehensive liquid chromatography coupled to photodiode array and mass spectrometry detection for complex real-world samples.
Marco Beccaria is a GC–MS specialist at Chromaleont s.r.l., c/o University of Messina, Italy. His research field is related to the development of LC–MS and LC×LC–MS methods for lipid studies in food and biological samples.
Paola Donato is an associate professor of analytical chemistry at the University of Messina, Italy. The main objectives of her research activity consist in the development of innovative LC techniques coupled to mass spectrometry (LC×LC–MS, LC×LC–MS–MS) for the analysis of highly complex mixtures.
Ivana Bonaccorsi is an assistant professor of food chemistry at the University of Messina, Italy. Her research interests include the study and characterization of complex natural matrices of food interest (derived Citrus, aromatic plant extracts, oils, etc.) by employing conventional and innovative chromatographic techniques.
Paola Dugo is a full professor of food chemistry at the University of Messina, Italy. Her research interests include the development of comprehensive liquid chromatography techniques and high throughput separation methods for the study of food components with potential biological activity.
Luigi Mondello is a full professor of analytical chemistry at the University of Messina, Italy. His research interests include chromatographic techniques, hyphenation to mass spectrometry, and multidimensional chromatographic approaches for the study of complex real-world samples.

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.









