An Ant-Man Perspective for Chromatography: My Stochastic World
I like both chromatography and Marvel movies, and an idea came to me as a result: Why not examine a chromatographic separation process from an Ant-Man perspective? I used the stochastic theory of chromatography to walk through this exercise. I am motivated to connect mathematics to separation science in an intelligible form, and convince every other chromatographer that deep down we all love mathematics. Please take a “random walk” with me through the column where chromatography is mathematics and mathematics is chromatography!
Because all chromatographers can operate a chromatograph, it is not obligatory to know how it really works inside. Nevertheless, I wanted to know what happens inside a column, and how and to what extent the analyte molecules interact with the stationary-phase surface.
There are several mathematical models used in chromatography. One of them is the stochastic theory that helps us understand the separation process at the molecular level. At first, when I was introduced to the stochastic theory, I disliked it because it seemed rather complex. But now, I am the one who wants to convince everyone that mathematics gives a great tool for us to be an “Ant-Man” and see what happens at the single molecule level. The stochastic theory is a sophisticated microscopic model that works as a magnifier; it gives us the number of the adsorption-desorption steps (n), and the average time that a molecule spends bound to the stationary phase (τ) from a single chromatogram!
The stochastic theory was first described by Giddings and Eyring more than 65 years ago (1). They realized that the analyte molecules wander randomly along the column. The stochastic theory uses probabilistic terms to represent the adsorption–desorption process. However, at the time, the computation methods were laborious, and the stochastic method could not be further developed. Afterward came the characteristic function (CF) method that simplified the representation of chromatograms (2,3). Shortly following the discovery of the CF method came the best realization of the chromatographic model—it does not depend on the separation procedure; thus, the CF method can be used for any type of chromatography. Regardless of whether it is reversed-phase liquid chromatography (RPLC), size-exclusion chromatography (SEC), ion-exchange chromatography (IEC), chiral chromatography, or another type of chromatography, we only need to consider the variables accordingly. This way, we can get information about the number of interactions between the stationary phase and the analyte molecule (n), and about the strength of this interaction (τ). Because these terms are easy to understand and work with, the stochastic theory should be preferred to macroscopic models. For example, band broadening phenomena of small molecules in high performance liquid chromatography (HPLC) columns are usually described by the general plate height model where a number of variables are used simultaneously.
Once we know in detail how a given separation process works, the characteristic function helps in the opposite way as well: The chromatogram can be depicted by inverse Fourier transform of the CF and the moments of the peak can be calculated. Thus, fundamental chromatographic characteristics can be tested. The first absolute moment is the retention time (tR), the second central moment is the variance (or the width) of the peak, and the third central moment holds information about the peak symmetry. In the simplest case, each moment can be calculated by the abovementioned two variables. When a novel model is developed, the characteristic function may become intricate, but the moments can still be calculated, and the chromatogram can be represented.
Usually, people are frightened because of the complex mathematics needed when using the stochastic theory, but nowadays we have powerful software packages, such as Wolfram Mathematica, and one can overcome the obstacles of performing Fourier transforms and inverse Fourier transforms. Starting now, our journey begins, and every chromatographic situation can be described.
In this article, I do not want to go into the fine details. This article provides a brief overview of our derived models. Thus, for details and more references, the reader is referred to our published studies in the literature (4–8). People usually dislike reading articles where a lot of equations are present; therefore, to accommodate reader preference, I have focused only the concepts and ideas of the models without using equations.
Over the years, I have developed, with my coworkers, three models for different chromatographic situations. These concern pore-size distribution in SEC (4), polydispersity in SEC (analyte molecule size distribution) (5), and interconversion during the separation process in chiral chromatography (8). Each model contains distributions that had to be introduced into the characteristic function to see what happens at the Ant-Man level of the molecules.
Pore-Size Distribution
Similar to Emmentaler (Swiss) cheese, which contains many different holes, stationary phase particles also contain a lot of pores. However, the actual size and shape of these pores are unknown. Inverse SEC (ISEC) can help to analyze the pore structure, but a proper model is needed to evaluate the data. Thus, we integrated pore-size distribution into the stochastic theory of SEC to get a universal and nondestructive method to determine the pore structure of any type of stationary phase by a single fitting procedure.
From destructive methods such as mercury intrusion or low-temperature nitrogen absorption, it is already known that the pore size is governed by a lognormal distribution. The lognormal distribution has two parameters: the expected value (or mean) of the pore size and the variance of the pore size. Because the characteristic function is in the Fourier domain, the mathematics degrade to multiplication, whereas in time domain we would need a convolution of the distribution and the inverse Fourier transformed CF, which would be cumbersome (convolution is a mathematical operation that is poorly known for many people). The moments of the peaks were calculated at several parameter combinations, and we realized that compared to the moments when no pore-size distribution is present, the new moments are multiplied by a factor that is actually the novel partition coefficient (K).
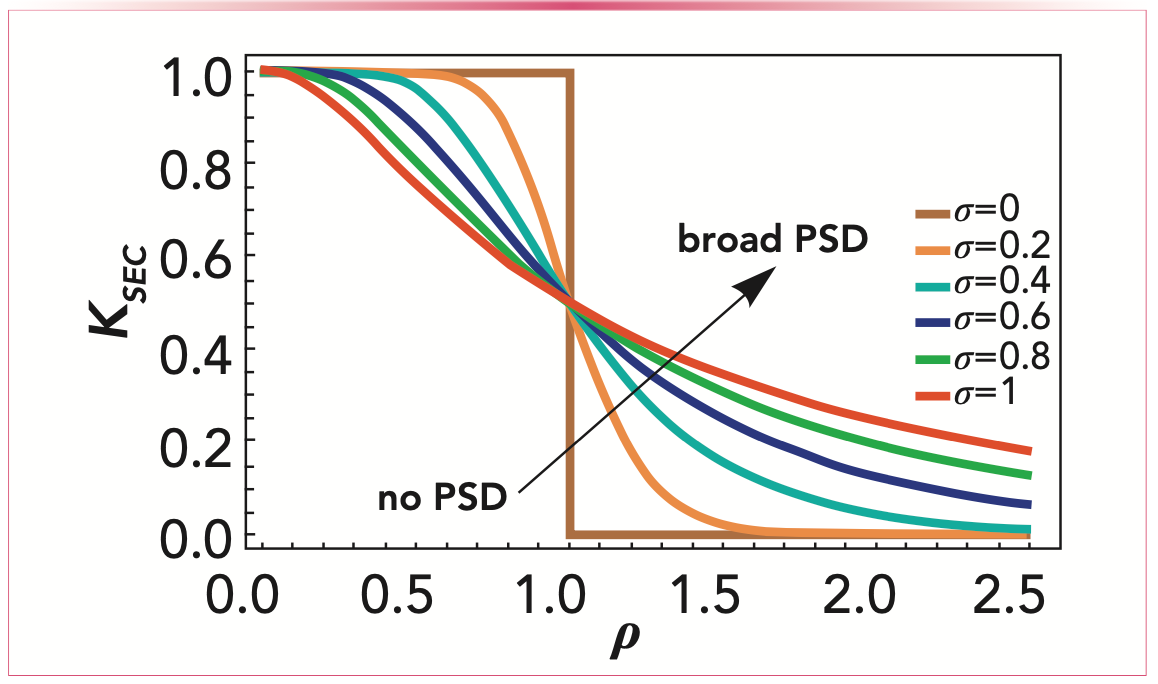
Regarding pore shape, we distinguished four different geometries: slit shaped, conical, spherical, and when no geometry is assumed (that actually gives the pure partition coefficient). By plotting K at several different parameter combinations, the model predicts how the molecules behave in either size-exclusion or when macromolecules are separated by any other type of chromatography. Figure 1 shows the partition coefficient when lognormal distributions of different pore sizes are assumed. This way, we obtained the relative pore accessibility. In an ideal case, when SEC is used, there are molecules that can enter the pores, and the fraction that is larger than the pore size is excluded. This is illustrated with σ = 0, when there is no distribution present. The other lines, where σ is increased, show that there is no sharp line between the molecules that can visit the pores and the ones that are excluded. There are pores that are big enough for larger molecules and that will cause wider peaks on the chromatogram.
FIGURE 1: Dependence of the partition coefficient on the pore size distribution. ρ is the ratio of the gyration radius of the analyte and the pore radius, σ is the variance of the pore size distributions (PSD). The broader the PSD, the more blurred the exclusion limit is.
Our model determined the pore structure of different commercially available stationary phases, both core–shell particles and monoliths (6,7). The novel stochastic model was able to determine that the pore geometry is rather spherical than conical for each investigated column. This geometric assumption gave better results near the exclusion limit than the previously used Knox model (9). There was an interesting discovery when the first- and second-generation monoliths (Chromolith Performance and the Chromolith High Resolution) were investigated: There is no difference in the real mean radii of the mesopore size, but the pore-size distribution is narrower in the latter case, and as a result, the pore size distribution is more homogeneous.
The application of the model is quite simple. The derived equations should be fitted by a proper software on the SEC calibration curve (the gyration radius against the retention volume) of the polymer standard molecules. We used a free, command-driven, function and data plotting program (Gnu-plot) for this purpose, but any other similar software will do the job.
Polydispersity
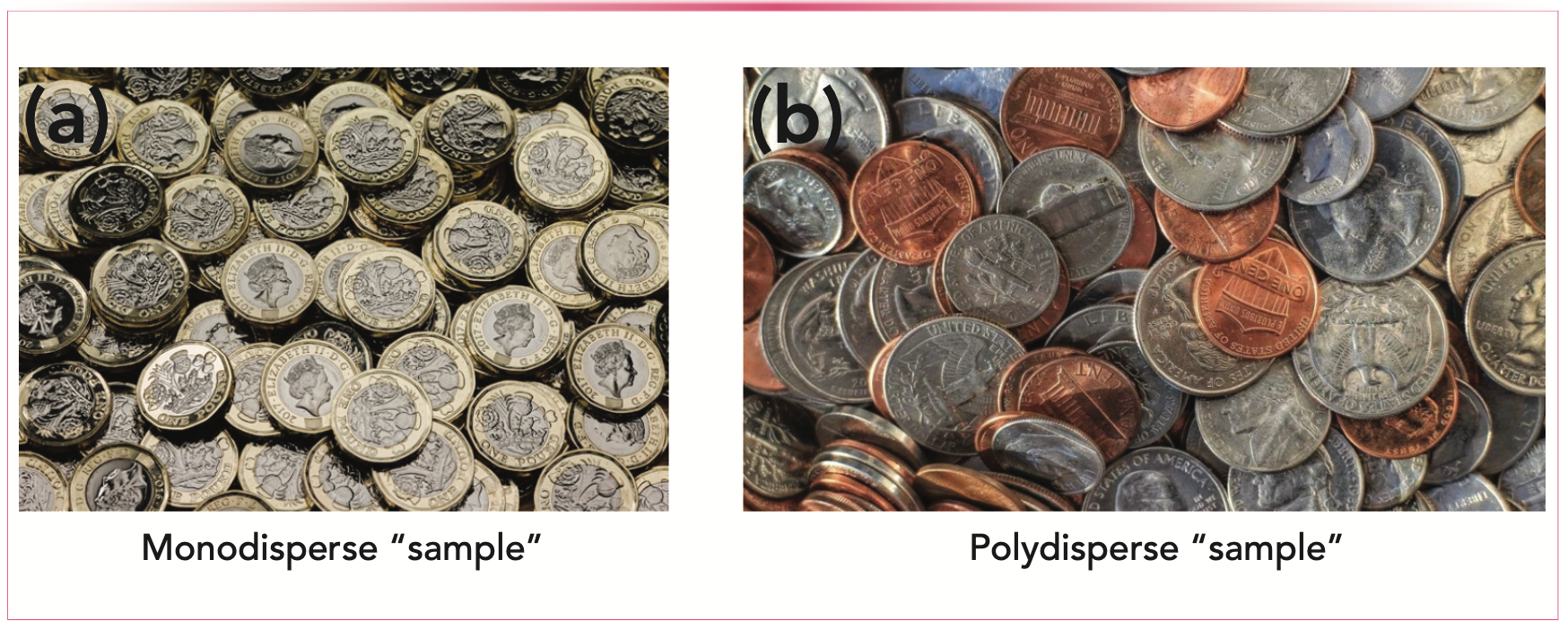
The polydispersity of the analyte is important mainly in ISEC or during the calibration step of SEC because it is important to know the size of the molecules injected onto the column to assess the pore size. If the standard molecule does not have a narrow molecular weight distribution (because of degradation, polymerization or simply by error in production), the size of the molecules can differ. The best way to illustrate the difference between a monodisperse and a polydisperse sample is to play with coins (Figure 2). Will these coins fit identically in the same hole?
FIGURE 2: The best way to depict what polydispersity means, showing the (a) monodisperse sample, and the (b) polydisperse sample.
The molecule size of the standards used in SEC and ISEC is governed by a normal distribution. The stochastic theory of SEC is adequate enough to integrate this information as well. Thus, the molecular size distribution was incorporated into the characteristic function, and a novel model was derived to describe this phenomenon. Chromatograms and their statistical moments were calculated for several pore geometries to investigate the effect of this distribution on the retention properties. It was concluded that the increase in polydispersity leads to a shift of retention time and causes a similar band-broadening to that of pore size distribution. The application of this model is, however, not feasible, since it would need a stationary phase having uniform pore size. Though the insights can be drawn by investigating the properties of the virtual chromatograms.
Our results verified previous observations and experiences: Both the pore-size distribution and the analyte polydispersity have a strong influence on the retention properties (retention time, peak width, and peak shape) of macromolecules. Because the wide pore size distribution will increase retention and efficiency for macromolecules, all modes of liquid chromatography that result in the efficient separation of macromolecules calls for a broad pore-size distribution.
Batman Peaks
Although the Batman movies are not part of the “Marvel Universe,” we have to talk about Batman peaks. Everyone knows Batman and his mask, but it is not common to know what we should think about when talking about Batman peaks in chromatography. If Batman partially hides below the baseline, one can see two ears and a headboard in between. The chromatogram of two enantiomers that interconvert into each other during the separation looks like what we see illustrated in Figure 3.
FIGURE 3: Batman-like figure superimposed on batman peaks.
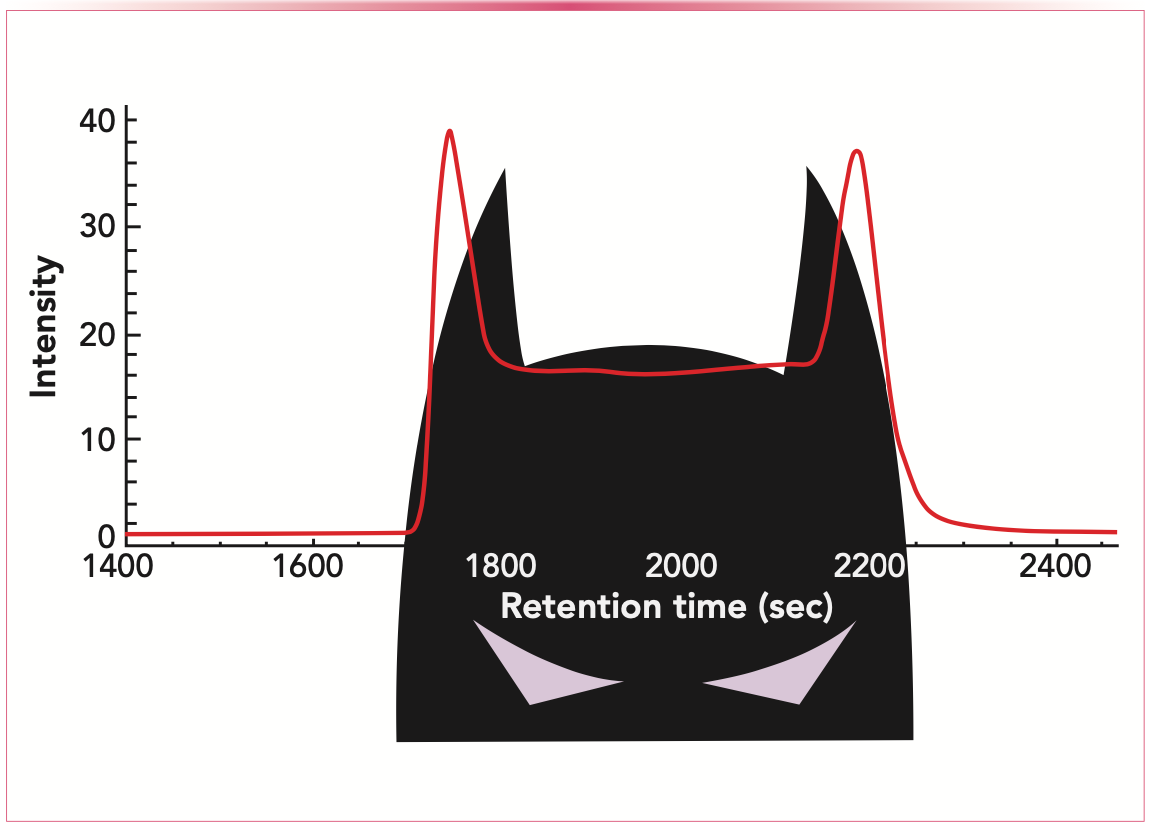
The pure enantiomers are separated, but in the plateau region, we observe molecules that interconverted a few times during the separation. The probabilities for the six different fractions (two fractions for the pure enantiomers that do not change during the separation, two fractions for the molecules with the same initial and eventual form and two fractions for molecules with different initial and eventual form) were already derived by Keller and Giddings in 1960 (10). The novel stochastic theory uses convolution of the proper distribution functions and the characteristic function. I would not say that the equations are open-and-shut, but Wolfram Mathematica can handle them.
Our novel model made it possible to examine the kinetics of the enantiomerization process from a series of chromatograms since the peak shape depends on the rate constant of this dynamic reaction. Applying our model is a true random walk along the column, because the different fractions need to be fitted separately. At first, the model was tested in silico, a number of chromatograms were simulated, and classical chromatographic properties were observed. Then, we investigated a commercially available drug molecule, quetiapine, that features a hindered ring-flip atropisomerism. The rate constant of the enantiomerization process was determined at a number of flow rates and temperatures. The interconversion rate constant can be determined from the slope of the fitted line when plotting the number of interconversions against time. Simultaneously, the rate constants were also determined at a number of temperatures from the optical activity of the two respective enantiomer fractions, and with the DCXplorer software derived by Trapp (11). Determined by the three different methods that were analyzed, a good agreement was found between the results.
This novel stochastic model is thus suitable to estimate the rate constant of interconversion from experimentally recorded data and gives better understanding to molecular interactions during the chromatographic separation. Moderate conditions are required because the separation circumstances (such as higher flow-rate, which causes temperature increase in the column) can influence the enantiomerization kinetics. Unfortunately, applying our model seems to be cumbersome because it requires the understanding of Fourier transform and inverse Fourier transform. To get through this obstacle, a software is in the process of being written by us that will allow the fitting procedure to be done by the computer automatically. This way, we would obtain a gentle and convenient method to determine rate constants from chromatograms if direct chiroptical measurement is difficult or unavailable. The application is, however, restricted to lower flow rates and temperatures where the enantiomerization process is limited to only a few interconversions. In the case of fast interconversion, the enantiomers cannot be separated and only one broad peak can be observed.
Although other types of interconversions can also result in Batman peaks, it is important to note that our model has even broader applicability. There is only one main restriction, which is that the molecule should have at most two forms that interconvert to each other. It is also possible that the transformation is limited to one of the directions.
Conclusion
The stochastic theory helps us to become an Ant-Man in the world of chromatography and envision the separation process at the molecular level using variables that are easy to understand. By considering different types of distributions, we have developed three individual novel models. Using them, the pore-size distribution can easily be ascertained in a nondestructive way from a number of chromatograms and when Batman peaks are present in chiral chromatography, the rate constant of the interconversion process can also be obtained.
So far, we have determined the pore geometry and the pore-size distribution of seven commercially available C18 stationary phases (both fully porous and core–shell) and the mesopore structure of monolithic columns of the first and second generation, respectively. ISEC combined with the novel stochastic model containing the pore-size distribution is a great nondestructive alternative to traditional methods such as mercury intrusion or low-temperature nitrogen adsorption. It is also possible to use this method to obtain the pore structure of any material.
The rate constant of the enantiomerization of quetiapine was determined by our novel stochastic model. Good correlation was found between these results and those obtained from optical activities and by using DCXplorer, respectively. Moreover, our model can help to understand the molecular processes emerging in chromatographic enantiomer separations. This stochastic theory can be used for other types of interconversion occurring during the separation process (tautomers and rotamers).
However, the stochastic theory gives us an infinite universe whose door we have just opened. Let’s be Ant-Men (and love math)!
Acknowledgments
I would like to thank the contribution of my colleagues: Attila Felinger, Ivett Bacskay, Gábor Németh, and Dóra Németh. Their help was essential to derive the models and apply them to real samples.
References
(1) J.C. Giddings and H.J. Eyring, Phys. Chem. 59, 416–421 (1955).
(2) F. Dondi, A. Cavazzini, M. Remelli, A. Felinger, and M. Martin, J. Chromatogr. A 943, 185–207 (2002).
(3) F. Dondi, G. Blo, M. Remelli, and P. Reschiglian, In Theoretical Advancement in Chromatography and Related Separation Techniques, F. Dondi and G. Guiochon, Eds. (Springer Netherlands, Dordrecht, The Netherlands, 1992), pp. 173–210.
(4) A. Sepsey, I. Bacskay, and A. Felinger, J. Chromatogr. A 1331, 52–60 (2014).
(5) A. Sepsey, I. Bacskay, and A. Felinger, J. Chromatogr. A 1365, 156–163 (2014).
(6) I. Bacskay, A. Sepsey, and A. Felinger, J. Chromatogr. A 1339, 110–117 (2014).
(7) I. Bacskay, A. Sepsey, and A. Felinger, J. Chromatogr. A 1359, 112–116 (2014).
(8) A. Sepsey, D.R. Németh, G. Németh, and A. Felinger, J. Chromatogr. A 1564, 155–162 (2018).
(9) J.H. Knox and H.P. Scott, J. Chromatogr. A 1984, 316, 311–332.
(10) A. Keller and J.C. Giddings, J. Chromatogr. A 3, 205–220 (1960).
(11) O. Trapp, J. Chromatogr. B 875, 42–47 (2008).
Annamária Sepsey is with the Department of Analytical and Environmental Chemistry at the University of Pécs in Pécs, Hungary. Direct correspondence to: sepsey@gamma.ttk.pte.hu.

Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
New Study Investigates Optimizing Extra-Column Band Broadening in Micro-flow Capillary LC
March 12th 2025Shimadzu Corporation and Vrije Universiteit Brussel researchers recently investigated how extra-column band broadening (ECBB) can be optimized in micro-flow capillary liquid chromatography.






