Ion Mobility–Mass Spectrometry (IM–MS): Enhancing Performance of Analytical Methods
Ion mobility–mass spectrometry (IM–MS) is a recently commercialized analytical technology that has great potential in addressing the challenges of complex sample analysis and providing an orthogonal separation dimension for detailed studies of ion structure. Ion mobility spectrometry and its combination with MS are introduced from the fundamental principles of collision theory and gas phase mobility separation. Modern IM–MS is a key separation technology for detailed molecular characterization studies and also as part of emerging data acquisition strategies for demanding small molecule and several applications.
Ion mobility spectrometry (IMS) is widely used as both an analytical separation approach and as a detector across a range of applications, including analyzing explosives (1), illicit drugs (2), pesticides (3), airborne vapors (4), and food ingredients (5). Understanding the principles of ion mobility as an analytical separation mechanism from an experimental standpoint requires an instrument capable of generating ions followed by pulsing or gating of ion packages into a gas-filled mobility cell where they are subject to displacement that is driven by an electric field. Unlike in mass spectrometry, where ions are transported and separated by various types of fields solely according to their mass-to-charge (m/z) ratio in a vacuum environment, IMS transport and separation of ions reflect the shape and size of the molecules, according to collisions of ions with a neutral drift gas, such as helium or nitrogen (6,7). Similar to other physical transport principles involving the application of electric fields, the characteristic property of the ions defining their transport behavior is referred to as ion mobility.
The transport of ions in this situation is summarized as follows: In the case of a weak electric field environment and considering only simple “hard sphere” collisions between ions and static drift gas molecules, a steady-state ion velocity (vd) is rapidly reached. The time taken for an ion to transient an IMS cell (drift time, td) of length L reflects this constant velocity. Fundamentally, the ratio of ion velocity in the drift cell to the applied electric field strength, E (ΔV/L), is referred to as the gas phase ion mobility, K (equation 1a), which may further be normalized to the experimental temperature (T) and pressure (p) to yield a reduced ion mobility, K0 (equation 1b) with respect to the National Institute of Standards and Technology (NIST) normal temperature and pressure (298.15 K, 101325 Pa) (8):
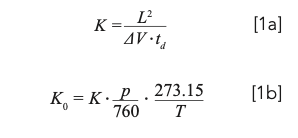
It should be noted that K0 is itself temperature-dependent and may also be influenced by the polarizability and purity of the drift gas (9,10).
Regarding the structural properties of ions, it is not possible to derive a true ion volume or surface area directly from IMS measurements, but the possibility of utilizing a momentum transfer integral model relating the apparent momentum transfer cross section or ion-neutral collisional cross section (Ω or CCS, m2) for a given ion to the gas phase mobility was proposed by Revercomb and Mason (6). This model (equation 2a) remains the best estimate to correctly derive a meaningful structural property from experimental measurements:
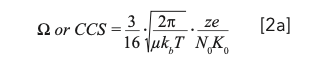
where e is the elementary charge (C), z is the integer charge of the ion, μ is the reduced mass of the ion-neutral pair (kg), kb is the Boltzmann constant (m2 kg/s2 K), N0 is the Loschmidt constant (1/m3).
In the case of drift tube ion mobility (DTIM), separations of small ions are experimentally performed in “drift” cells (or “drift tubes”) with uniform field strength applied along the axis of the cell. When the reduced field strength (in Townsend [Td] units) is experimentally considered “low” (that is, <15 Td, where 1 Td = 1 × 10-21 V m2), derivation of a new molecular descriptor (that is, the drift tube-derived CCS in a specified drift gas such as nitrogen; DTCCSN2) can be achieved from experimental measurements of p, V, T, and td using a version of the fundamental zero-field limit equation (equation 2a), sometimes referred to as the “Mason-Schamp equation” presented here in its expanded form (equation 2b) (6,11):
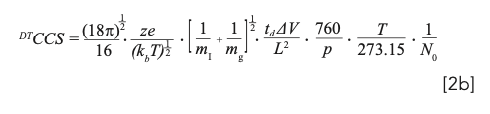
where mi and mg are the masses of the ion and drift gas, respectively (kg).
With this form of the fundamental equation, the orthogonality of IMS to MS is clear in that IMS measurements of isomeric and isobaric compounds can yield different arrival times, ion mobilities and, subsequently, derived CCS values to support their correct identification. Practically, it is noteworthy that determining CCS requires knowledge of the mass and charge state of the ion (or ions) of interest (equation 2b). This requirement is fulfilled with the combination of IMS with mass spectrometry (IM–MS), which is now a valuable analytical technology for broad applications addressing both the analysis of complex samples and as a tool for the structural characterization of ions (12,13). The addition of ion mobility as a dimension to existing analytical approaches including to liquid chromatography–mass spectrometry (LC–IM–MS) is now an emerging as an attractive platform to address both of these objectives (14).
Ion Mobility in Practice
Development of Instrumentation
The earliest descriptions of mobility separations of ions were reported in the 19th century with experiments describing the movement of ions through a gas under the influence of an electric field by Zeleny in 1898 (15) almost 15 years before the first descriptions of MS by Thomson (16). However, the principles of separation via ion mobility as an analytical technique was developed only in the early 1970s (7,17). In the case of a uniform field (drift tube) IMS measurements operating with low field strengths, the relationship between the applied field strength and the velocity of the ion is observed to be experimentally linear, which indicates that conditions of equation 2a are effectively satisfied for the application for the fundamental zero field equation. Although the range and validity of “low-field” conditions remains a topic of ongoing discussion within experimental and theoretical research (18), this type of measurement is currently agreed upon as the basis for a primary method for experimental IMS and IM–MS studies (19,20). The principles of ion mobility can also be experimentally exploited with alternative means including the use of high field strengths, nonstatic gas environments, and the application of nonuniform fields. Although these technologies require more complex physical models to fully elucidate mass transport phenomena, they have enriched the range of analytical ion mobility possibilities.
Types of IMS and Combination with Mass Spectrometry
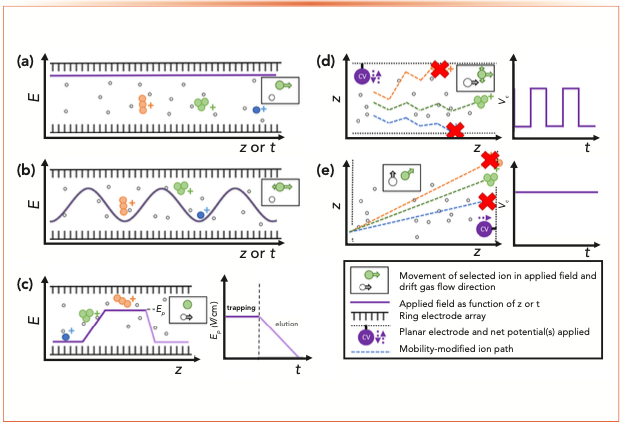
Several types of IMS have been transitioned to commercial technologies in recent decades, each having its own principle of operation and potential for combination with mass spectrometry. May and McLean (12) concisely categorize three main separation concepts employed, namely (a) time-dispersive methods, which generate an arrival time spectrum, with all ions drifting along the same pathway (that is, drift tube ion mobility spectrometry [DTIMS], and traveling wave ion mobility spectrometry [TWIMS]); (b) space-dispersive methods, which separate ions along different drift paths, based on their mobility differences (that is, field asymmetric waveform ion mobility spectrometry [FAIMS], differential mobility analyzer [DMA]); and (c) ion trapping followed by selective release, whereby ions are trapped within a pressurized region using a plateau electric field (Ep) with a counter flow of drift gas before being selectively ejected based on their mobility differences (that is, trapped ion mobility spectrometry [TIMS]). These different types of analyzers are schematically represented in Figure 1.
FIGURE 1: Schematic representation of operating principles of different IMS analyzers. (a) DTIMS; (b) TWIMS; (c) TIMS; (d) FAIMS; and (e) DMA.
The combination of IMS and mass spectrometry (IM–MS) was first reported by Cohen and Karasek (21) and applications first reported by Bowers and coworkers (22,23). Following the additional introduction of soft ionization methods, such as electrospray ionization (ESI) and matrix assisted laser desorption ionization (MALDI), using IM–MS for bioanalytical applications progressed rapidly (24–27). Hybrid IM–MS instrumentation was first made commercially available by Waters in 2006 with TWIM–MS technology (28), followed later by DTIM–MS (Agilent, TOFWerk) and TIM–MS (Bruker) during the following decade. During this period, numerous other standalone IMS devices that could be added to MS instruments based on both DMA and FAIMS were also commercialized.
Opportunities and Challenges
Untargeted Small Molecule Analysis
The combination of IM–MS with separation techniques such as LC is now a powerful platform for addressing the identity confirmation of unknown com- pounds within complex samples (14,29,30). Such “untargeted” workflows provide coverage of the broadest possible range of compounds by maximizing the selectivity, whereby transient separation properties (retention time), IM-derived parameters (CCS), accurate mass spectra, and high resolution mass fragment data can be used to provide a maximum number of identification points per putative compound (Figure 2).
FIGURE 2: Example of multiple identification point use for LC–IM–QTOFMS data. (a) All picked features from the low energy domain represented in tR, tA, and m/z space; (b) data representing an individual compound from a data independent acquisition; and (c) relevant data that can be used for library-based confirmation (underlined), and for alignment of transient signals (marked with asterisk).
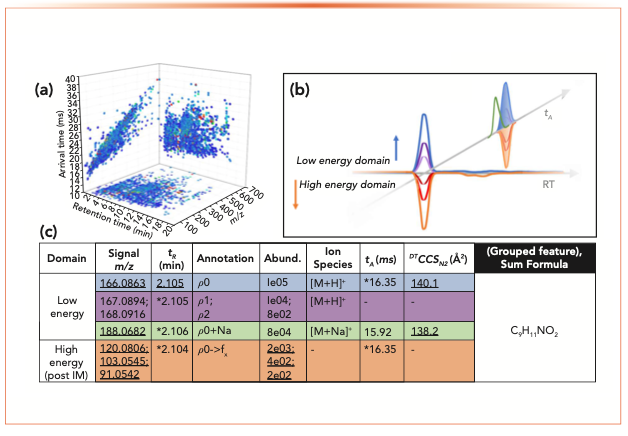
In contrast to the characterization of large biomolecules by IM–MS where multiply charged ions are revealed to exhibit more complex gas-phase conformational distributions, the separation of singly charged low molecular mass ions reveals that narrow uniform CCS distributions are observed for the majority of ions when using typical IM–MS conditions (31–36). This provides an opportunity to describe IMS or IM–MS in terms of classical chromatographic optimization parameters. Although the concept of retention is physically not of significance for IMS in comparison to chromatography, it is established that K and CCS depend on the relative sizes of the drift gas and analyte ion (equation 2b). Therefore, the selection of drift gas has a primary impact upon the ion mobility separation in terms of the observed drift times and potentially the selectivity. However, in contrast to electrophoresis performed in the liquid phase, selectivity cannot be easily modified for desolvated ions as the interactions of analytes with the neutral drift gas are weak. In terms of separation capacity and efficiency, it is of note that simplified peak capacity (37) and more advanced plate height theory models (38) may also be satisfactorily applied to describe the performance of some types of IMS. This type of theoretical treatment is particularly relevant for the optimization of standalone IMS for complex samples, but has a nuanced role for IM–MS whereby the separation of challenging isomeric and isobaric ions is of key interest for optimization.
Turning to opportunities with modern IM–MS, the additional IM separation prior to MS detection also allows for implementation of advanced data acquisition strategies. A key opportunity for IM–MS is pre- or post-IM application of energy to induce fragmentation of pseudomolecular ions (collision induced dissociation, or CID). Several commercial instruments share the possibility to perform post-IM CID experiments on the full m/z range emerging from the IM cell, representing a type of data independent acquisition (DIA) workflow whereby no duty cycle is expended on the successive isolation of individual precursors prior to fragmentation. Because of the preceding IM separation, an advantage is gained in reconciling fragment and precursor ions via drift (or arrival) time alignment performed during data processing (Figure 2). In contrast, using pre-IM CID is also possible on some of the commercial TWIM–MS instruments, which firstly allows IM information to be determined for fragment ions. Because this platform also allows for mass selection via a quadrupole, a narrow m/z range can be selectively isolated, fragmented and then submitted to the IM cell. Armed with these options, the potential for generating rich amounts of data from a single analysis is evident.
Finally, the utility of IM–MS as a routine analytical platform cannot rely solely on descriptions as a separation technology or direct comparisons to alternative MS data acquisition approaches. Instead, analytical validation of developed methods in terms of the ruggedness and performance for specified applications is required. Because high resolution mass spectrometry (HRMS) remains the primary technology for supporting identity confirmation for IM–MS platforms, the value of adding an ion mobility separation and particularly the use of CCS values must also be weighed against any problems that are simultaneously introduced for the evaluation of final datasets. Furthermore, as the complexity of the dataset increases with the inclusion of an additional separation dimension, this can also have drawbacks including a reduced working range because of the increased probability of system saturation, and decreased ion utilization efficiency finally impacting the limits of detection and quantification for analytical methods, which are critical parameters for the analysis of complex samples.
CCS for Identity Confirmation
One of the major benefits of employing IM–MS analytical methods for small molecules is the ability to calculate CCS values for unknown ions as they can be used as an identification point to support untargeted, targeted or screening workflows (Figure 2). However, unlike the situation for MS where monoisotopic molecular masses of ions can be considered as constants that can be directly calculated from tabulated values, calculations of true CCS values cannot be achieved with comparatively negligible uncertainty. This is a problem for the purposes of external calibration of IMS or IM–MS. This challenge can be illustrated by comparing the use of reference ions employed routinely for the external calibration of high-resolution MS, whereby the input uncertainties of calibrant m/z values are essentially zero and are complemented by experimental measurement uncertainties in the low ppm range. This allows routine mass errors of <5 ppm (or better) across a wide m/z range to be achieved and, subsequently, provides no hindrance to the comparison of accurate mass values measured on different types of HRMS. However, the inability to directly predict CCS values of calibrant ions via additive calculations remains a significant shortcoming with parallels to chromatographic retention time and retention indices being more appropriate.
Although fundamental IMS theory indicates that a perfectly characterized IMS cell should allow direct determination of td and subsequent calculation of the CCS of any ion in a given drift gas (equation 2b), this approach is characterized by large uncertainties associated with experimental parameters (p, T, E, and td), which lie in the low percent range (0.05–2%). Furthermore, in the case of IM–MS measurements, this “calibration-free” measurement is not possible when additional contributions to the drift time term are encountered (instrument dead time or detector rise time). For this reason, a primary method of measurement for low-field DTIM–MS provides a solution to equation 2b, whereby the field strength is stepped and arrival times (tA) recorded (Figure 3). Using a linear regression of the arrival times as a function of the inverse field strength, a correction term (t0) representing the time spent by the ion in the regions of the instrument other than the drift cell (or representing conditions of infinite field strength) is determined.
FIGURE 3: Experimental stepped-field arrival times for six reference ions as a function of applied field strength. Sources of uncertainty for the measurement and linear regression are propagated to the final determination of DTCCS. Adapted from (20).
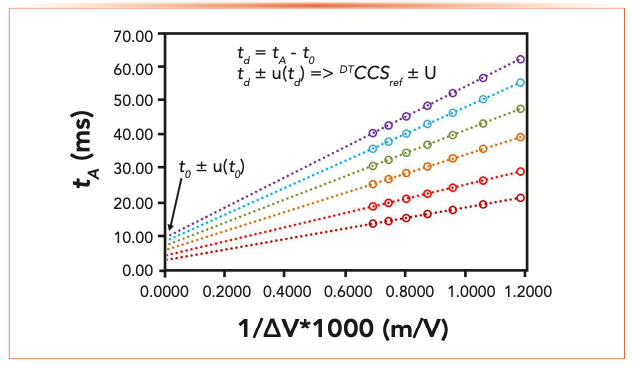
The stepped-field approach remains essential for IM–MS to demonstrate the linearity of the relationship between field strength and mobility (equation 1), and is used to provide reference DTCCS values used for subsequent secondary calibration. Indeed, all commercial IM– MS instrumentation is routinely externally calibrated using a measurement of calibrants under single set of conditions to allow a relationship between experimental arrival time and CCS to be defined for measurements of unknown ions. Currently, it is recommended that CCS values derived from measurements made using different IM–MS instrument types (Figure 1) be annotated in databases accordingly (DTCCS, TWCCS, TIMCCS) (19), but standardized calibrant sets and calibration procedures are still emerging topics despite the plethora of CCS data already published.
Although consensus on external calibration for IM–MS remains critical, there are further challenges on the horizon. First, the different nature of transport behavior experienced by ions in high-field strength environments, such as TWIMS and TIMS, may result in different gas-phase geometries being assumed by the ions. Second, the use of different ionization sources, source settings, solvents, and additives may affect compounds that have more than one proton acceptor site and, therefore, may yield multiple peaks for the same net ion formula (protomeric isomers, or “protomers”—a charge state positional isomers of a protonated molecule). Although these effects can be studied in carefully controlled experiments with analytical standards, this level of data interpretation is not currently feasible for routine applications addressing complex samples involving “untargeted” analyses. Such workflows with full scan IM–MS yield molecular information within several thousand compounds for a single sample. LC with IM–MS is a powerful approach for the discovery of new information and hypothesis generation, but the generation of huge numbers of CCS values for unknown ions and comparison of these to reference values from experimental or predictive CCS libraries is challenging. Furthermore, such comparisons are already hampered by the existing biases in calibrant CCS values, the bias and uncertainty contributions of the external calibration procedure employed, the experimental variability of measurement, and the influence of additional physicochemical factors that may arise on the platform used. For these reasons, further theoretical developments to better understand ionization phenomena along with experimental efforts toward unified calibration practices are needed.
Data Processing
Major commercial instruments have vendor-specific software for data visualization, CCS calibration, peak picking, serving identity confirmation and export for later statistical analysis. Many of the current challenges for improving IM–MS data processing follow similar trends observed for LC–MS when used as a true untargeted approach. In the case of IM–MS, the analysis of complex samples can have further negative influences on ion trapping processes as well as the mass detector as locally higher concentration in ion packages lead to an earlier onset of detector saturation. Although these issues can be circumvented by the use of multiplexing, this adds another step of data pre-processing to the overall workflow as data files must be demultiplexed prior to peak picking. Irrespective of the exact data acquisition strategy employed, application of IM–MS can also lead to an overestimation of the number of individual compounds because of signal artifacts, the additional separation of different charge states, adducts, and in-source fragments away from the primary ions used for confirming molecular formula. Finally, the development of advanced data processing workflows suitable for re-association of fragments with precursors for DIA workflows via a combination of retention time and arrival time alignment remains challenging, but progress is being made in this area and opportunities for addressing isotopic label tracing experiments are also emerging (39).
Conclusion
Overall, data processing of complex IM–MS datasets remains challenging for advanced research questions. Open-source software tools capable of pre-processing data and evaluating datasets have aimed to address individual issues during recent years, which will be an ongoing area of collaboration between analytical chemistry and informatics focused on molecule-level evaluation (“cheminformatics”) to deal with challenging research questions addressed using IM–MS technology for research and routine applications. Along with improving theoretical understanding and measurement standards for CCS determination, such developments can allow the full potential of IM–MS in small molecule and related fields to be unleashed.
References
(1) M. Tabrizchi and V. Ilbeigi, J. Hazard. Mater. 176(1), 692–696 (2010).
(2) J.R. Verkouteren and J.L. Staymates, Forensic Sci. Int. 206(1), 190–196 (2011).
(3) K. Tuovinen, M. Kolehmainen, and H. Paakkanen, Anal. Chim. Acta 429(2), 257–268 (2001).
(4) G.A. Eiceman, E.G. Nazarov, B. Tadjikov, and R.A. Miller, Field Anal. Chem. Technol. 4(6), 297–308 (2000).
(5) M. Hernández-Mesa, D. Ropartz, A.M. Garcia-Campana, H. Rogniaux, G. Dervilly-Pinel, and B. Le Bizec, Molecules 24(15), 2706 (2019).
(6) H.E. Revercomb and E.A. Mason, Anal. Chem. 47(7), 970–983 (1975).
(7) E.A. Mason and E.W. McDaniel, Transport Properties of Ions in Gases (Wiley, New York, New York, 1988).
(8) V. Gabelica and E. Marklund, Curr. Opin. Chem. Biol. 42, 51–59 (2018).
(9) Z. Karpas, Z. Berant, and O. Shahal, J. Am. Chem. Soc. 111(16), 6015–6018 (1989).
(10) T. Wyttenbach, G. von Helden, J.J. Batka, D. Carlat, and M.T. Bowers, J. Am. Soc. Mass Spectrom. 8(3), 275–282 (1997).
(11) E.A. Mason and H.W. Schamp, Ann. Phys. 4(3), 233–270 (1958).
(12) J.C. May and J.A. McLean, Anal. Chem. 87(3), 1422–1436 (2015).
(13) M.A. Ewing, M.S. Glover, and D.E. Clemmer, J. Chromatogr. A 1439, 3–25 (2016).
(14) V. D’Atri, T. Causon, O. Hernandez-Alba, A. Mutabazi, J.-.L Veuthey, S. Cianferani, and D. Guillarme, J. Sep. Sci. 41(1), 20–67 (2018).
(15) J. Zeleny, Lond. Edinb. Dublin Philos. Mag. J. Sci. 46(278), 120–154 (1898).
(16) J.J. Thomson, Lond. Edinb. Dublin Philos. Mag. J. Sci. 24(140), 209–253 (1912).
(17) E.W. McDaniel and E.A. Mason, The Mobility and Diffusion of Ions in Gases (Wiley, New York, 1973).
(18) B.C. Hauck, W.F. Siems, C.S. Harden, V.M. McHugh, and H.H. Hill, Int. J. Ion Mobil. Spectrom. 20(3), 57–66 (2017).
(19) V. Gabelica, A.A. Shvartsburg, C. Afonso, P. Barran, J.L.P. Benesch, C. Bleiholder, M.T. Bowers, A. Bilbao, M.F. Bush, J.L. Campbell, I.D.G. Campuzano, T. Causon, B.H. Clowers, C.S. Creaser, E. De Pauw, J. Far, F. Fernandez-Lima, J.C. Fjeldsted, K. Giles, M. Groessl, C.J. Hogan Jr., S. Hann, H.I. Kim, R.T. Kurulugama, J.C. May, J.A. McLean, K. Pagel, K. Richardson, M.E. Ridgeway, F. Rosu, F. Sobott, K. Thalassinos, S.J. Valentine, and T. Wyttenbach, Mass Spectrom. Rev. 38(3), 291–320 (2019).
(20) T. Causon and S. Hann, J. Am. Soc. Mass Spectrom. 31(10), 2102–2110 (2020).
(21) M.J. Cohen and F.W. Karasek, J. Chromatogr. Sci. 8(6), 330–337 (1970).
(22) M.T. Bowers, P.R. Kemper, G. von Helden, and P.A.M. van Koppen, Science 260(5113), 1446–1451 (1993).
(23) G. von Helden, M. Hsu, N. Gotts, and M.T. Bowers, J. Phys. Chem. 97(31), 8182–8192 (1993).
(24) S.J. Valentine, M. Kulchania, C.A.S. Barnes, and D.E. Clemmer, Int. J. Mass Spectrom. 212(1–3), 97–109 (2001).
(25) J.A. Taraszka, R. Kurulugama, R.A. Sowell, S.J. Valentine, S.L. Koeniger, R.J. Arnold, D.F. Miller, T.C. Kaufman, and D.E. Clemmer, J. Proteome Res. 4(4), 1223–1237 (2005).
(26) S.J. Valentine, M.D. Plasencia, X. Liu, M. Krishnan, S. Naylor, H.R. Udseth, R.D. Smith, and D.E. Clemmer, J. Proteome Res. 5(11), 2977–2984 (2006).
(27) E.S. Baker, B.H. Clowers, F. Li, K. Tang, A.V. Tolmachev, D.C. Prior, M.E. Belov, and R.D. Smith, J. Am. Soc. Mass Spectrom. 18(7), 1176–1187 (2007).
(28) I.D.G. Campuzano and K. Giles, TrAC Trends Anal. Chem. 120, 115620 (2019).
(29) T.O. Metz, E.S. Baker, E.L. Schymanski, R.S. Renslow, D.G. Thomas, T.J. Causon, I.K. Webb, S. Hann, R.D. Smith, and J.G. Teeguarden, Bionanalysis 9, 81–98 (2017).
(30) X. Zheng, R. Wojcik, X. Zhang, Y.M. Ibrahim, K.E. Burnum-Johnson, D.J. Orton, M.E. Monroe, R.J. Moore, R.D. Smith, and E.S. Baker, Annu. Rev. Anal. Chem. 10(1), 71–92 (2017).
(31) G. Paglia, J.P. Williams, L. Menikarachchi, J.W. Thompson, R. Tyldesley-Worster, S. Halldorsson, O. Rolfsson, A. Moseley, D. Grant, J. Langridge, B.O. Palsson, and G. Astarita, Anal. Chem. 86(8), 3985–3993 (2014).
(32) G. Paglia, P. Angel, J.P. Williams, K. Richardson, H.J. Olivos, J.W. Thompson, L. Menikarachchi, S. Lai, C. Walsh, A. Moseley, R.S. Plumb, D.F. Grant, B.O. Palsson, J. Langridge, S. Geromanos, and G. Astarita, Anal. Chem. 87(2), 1137–1144 (2015).
(33) S.M. Stow, T.J. Causon, X. Zheng, R.T. Kurulugama, T. Mairinger, J.C. May, E.E. Rennie, E.S. Baker, R.D. Smith, J.A. McLean, S. Hann, and J.C. Fjeldsted, Anal. Chem. 89(17), 9048–9055 (2017).
(34) X. Zheng, N.A. Aly, Y. Zhou, K.T. Dupuis, A. Bilbao, V.L. Paurus, D.J. Orton, R. Wilson, S.H. Payne, R.D. Smith, and E.S. Baker, Chem. Sci. 8(11), 7724–7736 (2017).
(35) C.M. Nichols, J.N. Dodds, B.S. Rose, J.A. Picache, C.B. Morris, S.G. Codreanu, J.C. May, S.D. Sherrod, and J.A. McLean, Anal. Chem. 90(24), 14484–14492 (2018).
(36) V. Hinnenkamp, J. Klein, S.W. Meckelmann, P. Balsaa, T.C. Schmidt, and O.J. Schmitz, Anal. Chem. 90(20), 12042–12050 (2018).
(37) T.J. Causon and S. Hann, J. Chromatogr. A 1416, 47–56 (2015).
(38) M. Grabarics, M. Lettow, A.T. Kirk, G. von Helden, T.J. Causon, and K. Pagel, Analyst 145(19), 6313–6333 (2020).
(39) M.L. Feuerstein, R.T. Kurulugama, S. Hann, and T. Causon, Anal. Chim. Acta. 1163, 338508 (2021).
Tim Causon is with the Institute of Analytical Chemistry at the University of Natural Resources and Life Sciences in Vienna, Austria. Direct correspondence to: Tim.Causon@boku.ac.at.

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)






