AFFF-MALS-RI for Determining the Mass and Size Distributions of Amylose and Amylopectins in Starch
The Application Notebook
Integration of RI peak areas enabled calculation of the AMY:AMP ratios, in excellent agreement with the nominal values. The values for Mw and Rz fall within the generally accepted limits found in the literature. Conformational plots for the AMP component verify its branched nature.
Starch is used for a variety of industrial and nutritional purposes. Its functional properties are influenced by the ratio and molar masses of its macromolecular constituents, which vary with source, crop year, and climate. Starch contains large homopolymers of amylose (AMY) and amylopectin (AMP).
Linear AMY consists of long chains of (1â¨4)-α-D-glucose linkages, while the higher molar mass AMP is a branched structure containing a mixture of (1â¨4)-α- and (1â¨6)-α-D-glucose linked residues. The goal of this work was to apply AF4-MALS-RI to separate AMY and AMP in order to calculate a mass ratio, to determine the molar mass distributions, the average molecular weights (Mw), and the mean-square radius (Rz) of the AMP component. We applied the technique to starches with AMY:AMP ratios covering a wide range.
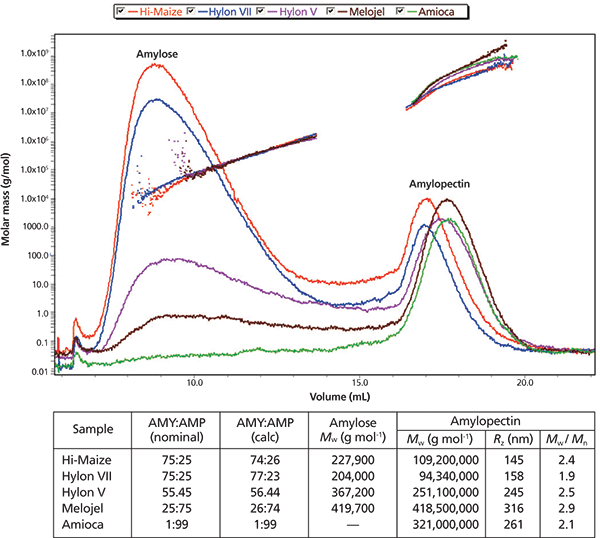
Figure 1: AF4-MALS-RI results for five native starches of varying AMY:AMP ratio: AF4-RI fractograms with molar mass distributions overlaid. (Cross-flow (Vx) = 1.0 to 0.1 mL/min in 10 min, then Vx = 0.0 mL/min.)
An Eclipse AF4 system (Wyatt Technology) was equipped with a short (18 cm) channel, a 350 μm spacer, and a regenerated cellulose (10 kDa cutoff) membrane. Detection was accomplished with DAWN Multi Angle Light Scattering (MALS) and Optilab RI detectors (both instruments Wyatt Technology). The channel flow was maintained at 1.0 mL/min and the cross-flow was varied linearly from 1.0 to 0.1 mL/min for 10 min, then abruptly switched to 0.0 mL/min.
Integration of RI peak areas enabled calculation of the AMY:AMP ratios, in excellent agreement with the nominal values. The values for Mw and Rz fall within the generally accepted limits found in the literature. Conformational plots for the AMP component verify its branched nature.
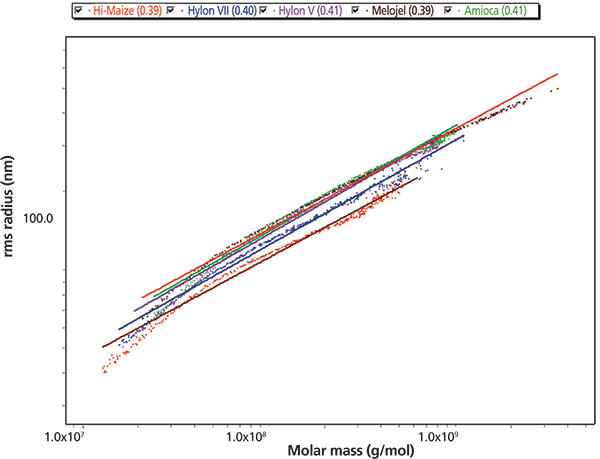
Figure 2: Conformation plot (log Rz versus log Mw) for the amylopectin component of five starches (slopes 0.39-0.41 indicative of branching).
This note graciously submitted by Rick White and Eija Chiaramonte, Global Analytical Sciences-Personal Health, The Procter & Gamble Company, Mason, OH.
DAWN®, miniDAWN®, ASTRA®, Optilab® and the Wyatt Technology logo are registered trademarks of Wyatt Technology Corporation. ©2013 Wyatt Technology Corporation 4/4/13.

Wyatt Technology
6300 Hollister Avenue, Santa Barbara, CA 93117
tel. +1 (805) 681-9009, fax +1 (805) 681-0123
Website: www.wyatt.com

Determining Enhanced Sensitivity to Odors due to Anxiety-Associated Chemosignals with GC
May 8th 2025Based on their hypothesis that smelling anxiety chemosignals can, like visual anxiety induction, lead to an increase in odor sensitivity, a joint study between the University of Erlangen-Nuremberg (Erlangen, Germany) and the Fraunhofer Institute for Process Engineering and Packaging (Freising, Germany) combined behavioral experiments, odor profile analysis by a trained panel, and instrumental analysis of odorants (gas chromatography-olfactometry) and volatiles (gas chromatography-mass spectrometry).
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)

















