Advantages of Using Nitrogen in Capillary GC
LCGC North America
The most prominent advantage of using nitrogen as a carrier gas is that it is the most efficient one when used at its optimum linear velocity.
The most prominent advantage of using nitrogen as a carrier gas is that of all the carrier gases it is the most efficient when used at its optimum linear velocity (12 cm/s), meaning it will produce the narrowest chromatographic peaks. However, you may be thinking that at that linear velocity analyses are going to be much slower compared to helium and hydrogen (whose optimum linear velocities are 35 and 40–50 cm/s, respectively-see Figure 1). And you would be right, operating at the optimum linear velocity for nitrogen will result in a longer analysis. However, you will be pleased to know that there are ways to remedy this problem.
Figure 1: Van Deemter plots with nitrogen, helium, and nitrogen carrier gas.
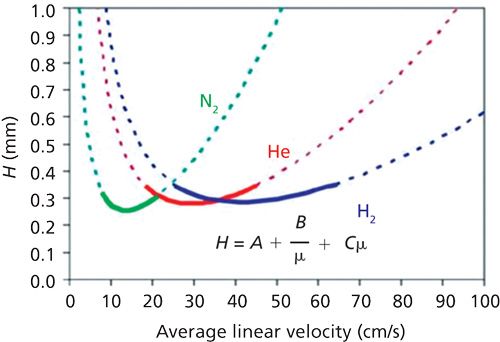
If you operate at slightly higher than the optimum linear velocity then you can reduce analysis time, although you will lose some efficiency (if your method has excess resolution then this loss will not be a problem). Typically method conversion can be much simpler than with hydrogen, because the same column and temperature program can be used. In fact, you can simply take existing helium-based methods that have sufficient resolution and translate them directly to nitrogen by using the same column and leaving everything including the head pressure the same. You will obtain exactly the same chromatogram in exactly the same time with slightly broader, less intense (30%) peaks (again, if you are able to work with slightly reduced sensitivity then this peak shape difference should not be seen as a problem). If you are carrying out a temperature-programmed method in constant flow mode, the impact of linear velocity on efficiency is also much reduced.
Of course methods using nitrogen can be optimized to produce faster analyses without sacrificing the big advantage of efficiency. Efficiency losses can be compensated for by using a column with a shorter, narrower internal diameter. As column internal diameter decreases, the optimum linear velocity increases (regardless of carrier gas), producing flatter van Deemter curves; ultimately this result means that you can operate at linear velocities above optimum without losing efficiency. Analysis time can be maintained by adjusting the linear velocity to match the holdup time to the original method. Matching the phase ratio (β) of the original and new column will preserve the selectivity of the separation. When altering column dimensions 20 m columns work well to convert methods which originally used 30 m columns and 0.15- or 0.18-mm i.d. columns are a practical narrow-bore column that will not usually require any adaptations to the instrument, such as the use of smaller-volume inlet liners. It should be noted that there will be a loss of capacity with smaller internal diameter and shorter length columns, which could lead to column overload if it is not taken into account. Finally, selectivity has the greatest impact on chromatographic resolution and the parameter that most greatly influences selectivity is phase chemistry, therefore, loss of efficiency can be compensated for by using application specific selective phases (for example, pesticides, FAME).
Another advantage of nitrogen is that it can be generated in situ using generators. The nitrogen is generated directly from air using membrane filters or carbon molecular sieve to remove oxygen, water, and other impurities. This capability makes nitrogen readily available, less expensive, and safe.
Ultimately nitrogen is suitable for simple routine analysis-with nitrogen methods being primarily aimed at gas chromatography-flame ionization detection (GC–FID) methods (7 out of 10 instruments). Two-dimensional (2D) GC is also ideally suited to nitrogen because resolution issues are solved by using two different columns. Nitrogen carrier gas has limited applicability to GC–mass spectrometry (MS) applications, but it is suitable for analyses where high sensitivity is not needed. It does, however, require modifications to the tune parameters to optimize MS performance. Some other pulse discharge–based detection systems prefer helium because of the detection principle and will not be suitable for nitrogen-based methods.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.













