A Simple and Interactive Column Classification for Reversed-Phase Liquid Chromatography: The Carotenoid Test, Part II: Additional Studies and Practical Use of the Classification Map
LCGC North America
The carotenoid test allows one to build a simple classification map of stationary phases used in reversed-phase liquid chromatography, on the basis of the shape recognition(plotted on the x axis) the polar surface activity(plotted on the y axis) and the phase hydrophobicity (related by the bubble size).
The carotenoid test allows one to build a simple classification map of stationary phases used in reversed-phase liquid chromatography, on the basis of the shape recognition (plotted on the x axis), the polar surface activity (plotted on the y axis), and the phase hydrophobicity (related by the bubble size).
We have shown that shape recognition, which indicates the ability of the stationary phase to separate closely related compounds such as isomers, mainly depends on the bonding density, the pore size, and the ligand type (mono- or polyfunctional). The polar surface activity relates to the ability of polar compounds to interact with residual silanols or ligand polar groups. These interactions mainly depend on silica precoverage by polymers, the use of hybrid silica, endcapping treatments, or surface polymerization and are of a great importance for basic drugs. Hydrophobicity depends on both the specific surface area and the bonding density. In this second part of the “Column Watch” series, additional studies were conducted to show more detail and demonstrate the usefulness of this classification map: Does the evolution of the stationary phases provide real changes in their chromatographic behavior? What is the effect of using hybrid silica supports? Are the stationary phases bonded on superficially porous particles identical and do varied ligand chemistries provide specific behaviors (phenyl-hexyl, cholesteryl)? The use of the map to study stationary phases based on sub-2-µm particles will also be discussed. Moreover, the open interactive web interface is presented. Columns can be individually selected to provide a rapid look at their position on the map, and other columns can be added for comparisons. Several icons allow one to directly compare different columns from a single manufacturer or from several manufacturers. Finally, this tool is very convenient for a rapid selection of identical or different stationary phases on the basis of the three studied properties.
In Part I of the series, the fundamentals of the classification map were described, as well as the relevance of the chromatographic parameters used to build this map (1). We studied the effect of endcapping treatments, bonding density, and pore size differences on the three studied properties: hydrophobicity, shape recognition, and polar surface activity. Some questions about other possible approaches, principal component analysis and ranking (2), were also addressed to improve understanding of the results obtained in each case.
Additional Studies
The classification map based on the carotenoid test can also be use to follow the advancements in stationary phase design, differences, and similarities of superficially porous particle (SPP) phases, of sub-2-µm fully porous particle (FPP) columns, and varied ligand chemistries.
Phase Evolutions
Figure 1 displays the location of different versions of some phases. Some columns from the same brands are located very close to each other: for instance Gemini C18 (n°127) and Gemini NX (n°156) (both from Phenomenex); and Chromolith RP 18e (n°29) and HR RP 18e (n°211) (both from Merck Millipore (3). Only slight changes are noticeable. The only observable difference for the Gemini phases is that the hydrophobicity of Gemini NX (n°156) is lower than that for Gemini C18 (n°127) as indicated by the smaller bubble size. For the two Chromolith phases, Chromolith RP 18e (n°29) and Chromolith HR RP 18e (n°211), which differ in the meso and macro pore sizes, the chromatographic behavior seems rather unchanged, indicating that the bonding characteristics are similar. Identical conclusions can be done for the series Atlantis dC18 (n°120), Atlantis T3 (n°153), and XSelect HSS T3 (n°188) (all three from Waters) in group 1 or for Zorbax Eclipse (n°63) and Eclipse plus (n°138), Pursuit (n°119), and Pursuit Rx (n°135) (all from Agilent Technologies) in group 9.
Figure 1: Location of various versions of stationary phases on the classification map.
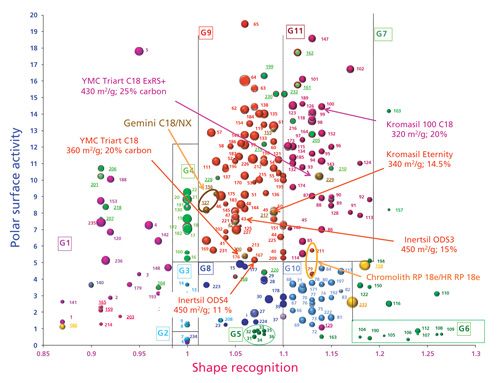
However, the changes are more significant between some phase versions within a single brand. For instance, YMC C18 ExRS+ (n°229) displays a higher value for the shape recognition and a lower polar surface activity compared to the YMC-Triart C18 (n°200) (both from YMC America), both of these features are related to a higher bonding density (higher carbon content for almost identical specific surface area). Conversely, the decrease in the carbon content from Kromasil 100 C18 (n°100) to Kromasil Eternity (n°217) (both from Sigma-Aldrich by Eka Nobel) leads to a lower value for the shape recognition and a higher polar surface activity, again related to a decrease in the carbon content with close specific surface area. Inertsil ODS-3 (n°43) and ODS-4 (n°167) (both from GL Sciences) are bonded on the same silica, and both are endcapped. The decrease in the carbon content (from 15 to 11%) decreases hydrophobicity (as appears in the bubble size) and increases the polar surface activity, as could be expected. The shape recognition is almost unchanged.
These results show that some developments of stationary-phase designs, perhaps meant to improve stationary-phase stability (different bonding chemistry or silica, or different bonding pathway) within one brand do not necessarily provide changes in the chromatographic behavior. These evolutions are usually not explained by manufacturers. In other cases, the advancements lead to different chromatographic behavior. These stationary phases should be considered as chromatographically different, leading to potential retention and selectivity changes.
Superficially Porous Particles
Figure 2 displays the location of the phases bonded on SPPs, also called core–shell particles (4). It is now well recognized that the efficiency achieved with SPP particles having a particle diameter of approximately 3 µm, is close to the efficiency achieved with sub-2-µm FPPs. However, the back pressure generated by 3-µm SPPs is dramatically lower than the pressure drop caused by sub-2 µm FPPs (with comparable column dimensions). Nevertheless, these C18-bonded SPP phases are not all located in the same area of the map. Nine of the SPP phases display a low polar surface activity: Cortecs C18 (n°219) (Waters), YMC Meteoric Core (n°230), Poroshell 120 ec-C18 (n°199) (Agilent Technologies), Cosmocore C18 (n°232) (Nacalai), Halo C18 (n°162) (Advanced Materials Technology), Ascentis Express C18 (n°161) (Sigma-Aldrich), Sunshell C18 (n°216) (ChromaNik Technologies), Nucleoshell RP C18 (n°205) (Machery-Nagel), and Accucore C18 (n°210) (Thermo Fisher Scientific).
Figure 2: Location of superficially porous particle C18 phases on the classification map.
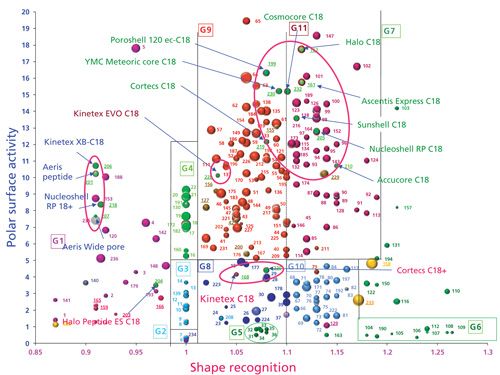
Located in group 9, we also find the Kinetex EVO C18 (n°137) (Phenomenex), which exhibits a different shape recognition compared to the other nine phases. This phase is bonded on a hybrid silica, possibly leading to a lower bonding density and explaining the shift in the 13-cis/all-trans β-carotene selectivity toward values close to 1. In fact, the Kinetex EVO phase is very close to the Gemini NX (n°168), which is bonded on the same type of hybrid silica (and probably uses the same type of synthesis process to bond the ligand). Four columns are located in group 1: Kinetex XB-C18 (n°206) (Phenomenex), Aeris Peptide (n°201) (Phenomenex), Aeris Wide Pore (n°207) (Phenomenex), and Nucleoshell RP 18+ (n°218) (Machery-Nagel), which represent a retention inversion of cis/trans-β-carotene, showing a specific behavior in shape recognition. This behavior is close to the one of the Atlantis dC18, T3, and XSelect HSS T3 phases, which possess multiple linkages between the C18 chain and the silica surface. The Halo Peptide ES C18 phase (n°204) (Advanced Materials Technology) is also located in this group 1, but displays a higher polar surface activity. Kinetex C18 (n°168) (Phenomenex) and Cortecs C18+ (n°220) (Waters) also have a rather high surface polar activity, but are located in group 8, containing monofunctional phases with a low bonding density. When comparing the two Cortecs, C18 and C18+, one can see that they display the same shape recognition, indicating an identical bonding density. The difference in the polar surface activity could be due to the polar positive charge on the silica surface of the C18+. Besides, the Cortecs C18 is very close to the XBridge C18 (n°155) (Waters), showing that one can shift from the one (FPP) to the other (SPP) with a minimal change in retention and selectivity. To conclude, these SPPs are spread at least in three areas having different chromatographic behavior, showing that the choice of SPP columns should be also based on their chemical properties.
Ultrahigh-Pressure Liquid Chromatography Phases
Figure 3 displays the effect of the particle size change on the chromatographic behavior of the columns. For Luna C18 2 (n° 52 and n° 196) (Phenomenex), Nucleodur Isis (n°131 and 195) (Machery-Nagel) or Uptisphere Strategy C18-2 (n°137 and 195) (Interchim), there are no significant differences between the 5- and the 2.5-, 2.2- or 1.9-µmparticles. As expected, and fortunately, the particle size change does not modify the bonding density and the amount of residual silanol groups, leading to identical values for the studied selectivities. Nucleodur Isis C18 is located in group 7 because of its horizontal polymerization in which the chains are linked to each other close to the silica surface. On the other hand, for the Synergi phases (Phenomenex): Max-RP (n°160 and n°191), Fusion (n°179 and n°193), and Hydro (n°178 and n°192), one can see some location differences, mainly for the shape recognition for Fusion and Hydro. The decrease in the particle size is related to an increase in the cis/trans-β-carotene separation factor, indicating an increase in the apparent bonding density. This may be a result of an additional change of the pore size, equal to 80 Å for the 5-µm particle and 100 Å for the 2.5-µm columns. As described in part I of this column series, the increase in pore curvature, related to the pore diameter, could induce a slightly different tridimensional chain organization, with a subsequent change in shape recognition. This phenomenon does not occur for the Synergi Max-RP phase because of the small chain length (C12), which is unable to achieve the separation of the two studied isomers, whatever the pore size.
Figure 3: Location of stationary phases bonded on different particle sizes and of brands used in UHPLC on the classification map.
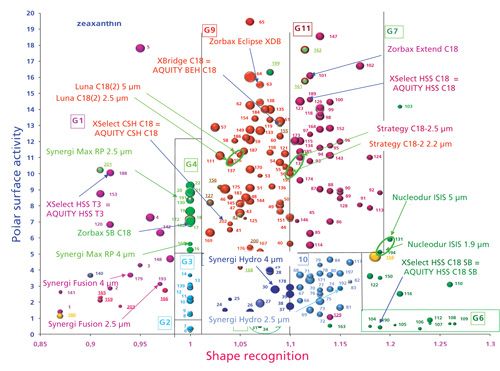
Figure 3 also presents the location of some stationary phases having different names depending on their particle size, such as for high performance liquid chromatography (HPLC) or ultrahigh-pressure liquid chromatography (UHPLC) particles. The decrease in the particle size from 5 to 1.7 or 1.8 µm allows one to reach very high theoretical plate numbers. Both the eddy diffusion and the resistance mass transfer are lower with small particles, but the use of small particle size also requires very high pressure, in relation with the Darcy’s law, which shows that pressure drop varies inversely with the square of the particle diameter. However, the bonding chemistry for particles having different sizes is unchanged. Their chromatographic properties based on interactions, such as shape recognition, polar surface activity and hydrophobicity, are not related to this change in the particle size. Consequently, the location of the stationary phases devoted to UHPLC should be identical to the one of the phases used for HPLC. By the way, for some brands, the name of the phase is unchanged whatever the particle size; for instance, Zorbax XDB (n°63), Zorbax Extend (a bidentate ligand) (n°101), and Zorbax SB C18 (n°17) (all from Agilent Technologies) have the same name whatever the particle size. For other brands, the name of the phases is different depending on the particle size. For example, the Waters phases are called Acquity for UHPLC particles, and XSelect and XBridge for classical HPLC particles. In that case, the location of the UHPLC phase is provided by the name of the HPLC phases, XBridge (n°155) or XSelect (CSH C18 n°202; HSS T3 n°188; HSS C18 n°189) instead of Acquity.
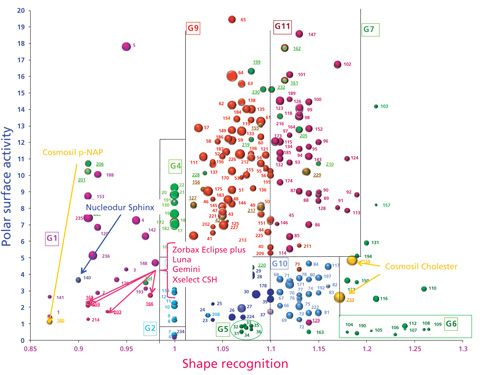
The carotenoid test can also be applied to other ligand chemistries, such as naphthyl, phenyl-hexyl, and chlolesteryl. As seen in Figure 4, the naphthyl group, Cosmosil πNAP (n°180) (Nacalai) and the phenyl-hexyl bonded phases: Zorbax Eclipse Plus (n°165) (Agilent Technologies), Luna (n°159) (Phenomenex), Gemini (n°166) (Phenomenex), and XSelect CSH (n°203) (Waters) are located in the same group due to a specific shape recognition (13-cis-β-carotene is eluted before the all-trans isomer). Moreover, these phases also display a high polar surface activity, likely due to accessible residual silanol groups on the silica surface. A mixed ligand surface, which includes both C18 and phenyl parts, is also located in this group (Nucleodur Sphinx; n°140, Machery-Nagel). Finally, the cholesteryl group, Cosmosil Cholester (n° 158 and 233) (Nacalai) is at the opposite side of the map due to increased shape recognition. This chromatographic behavior is identical to polyfunctional bonded phases (group 6 and 7). Cholesteryl phases also display very high hydrophobicity, as shown by the large bubble sizes.
Figure 4: Location of naphthyl, phenyl-hexyl, and cholesteryl ligand on the classification map.
The Interactive Interface
The classification map containing more than 250 stationary phases is accessible on the web site of ICOA at the following link: http://www.icoa.fr/classification_c18_columns/index.php. On the main page, the map is on the left where each point repesents a column (Figure 5), and each color corresponds to one of the groups in which the columns are located. On this main page, the icons to select columns are on the right (Figure 6). Two tabs called “User guide” and “Information” and provide useful information about the use of the intuitive interface.
Figure 5: Classification map on the interactive web version of the classification version.
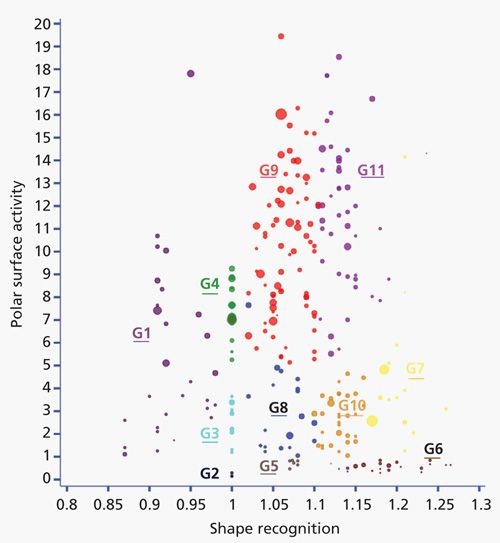
Figure 6: Picture of the selection icons on the main menu of the interactive web version of the classification map.
Briefly, the interface allows varied views and comparisons of the column information. The list of columns characterized is available on pages 1, 2, and 3. Each column can be selected by clicking on the column name icon, and the column appears onto the map as a colored point (pink), whereas all the others are colored in gray. The size of point of the selected column is now enlarged to allow a better focus on the location of the point, and consequently this size does not anymore correspond to the column hydrophobicity. Additional columns can be individually selected for comparison of their location (that is, their chromatographic behavior), and these additional points are also colored in pink, with the same size.
Other views can be generated by using tabs Manufacturer, Groups, Ligands, or Particle types. When selecting one manufacturer, for example, all the phases from this manufacturer appear in pink both on the map and on the list of columns, then another manufacturer can be selected (the new points appear in green) that favors a rapid and simple comparison between the two sets of columns. The column research can also be refined by choosing the superficially porous particles from all the phases of one vendor, and so on. At each step, a “Reset” tab (which appears in red after the first selection) allows one to get back to the main menu.
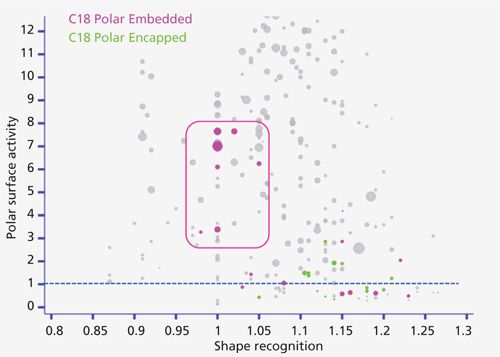
One can compare the properties of varied ligand chemistries-for instance, between the polar-embedded C18 and the polar-endcapped C18 phases. The polar-embedded ligands were mainly developed to work with water-rich mobile phases and to be used with wide elution gradients of organic solvent. It was recognized that in water-rich mobile phase the very hydrophobic C18 chains tend to collapse, thereby dramatically reducing the analyte retention. This collapse behavior should be avoided with polar-embedded or endcapped bonded phases because of the interaction between water and the polar groups close to the silica surface. However, when using a supercritical mobile phase composed of carbon dioxide and methanol, zeaxanthin is strongly retained by these polar groups that often lead to a retention inversion between zeaxanthin and all-trans β-carotene, such as to a separation factor lower than one.
As shown in Figure 7, many of these phases (polar endcapped and polar embedded) are located below a value of 1 on the y axis, indicating high polar surface activity of these polar groups. Besides, as expected, most of the polar embedded phases display low hydrophobicity (the bubble sizes are small), and for some of them a high shape recognition, as described elsewhere (5–7). Other polar-endcapped phases are located in groups 1, 3, 4, and 9, with a lower polar surface activity and a higher hydrophobicity.
Figure 7: Location of polar-embedded and polar-endcapped stationary phases on the interactive web version of the classification map.
Conclusion
In conclusion, this interactive classification map is a powerful tool that provides useful knowledge about the properties of bonded stationary phases used in HPLC with easy comparison. It offers various ways of selection, including by the column name, the column manufacturer, the ligand, and the particle type. It allows one to highlight the specific behavior of phenyl-hexyl and cholesteryl ligands, which display opposing shape recognition, and hydrophobicity, or polar-embedded phases that display a chromatographic behavior close to polyfunctional C18 phases. It also shows the large repartition of the superficially porous particles into the space of properties (varied shape recognition and polar surface activities.
The close study of the point location is also informative about the effect of horizontal polymerization and the presence of charges at the silica surface. Low hydrophobicity of C18 phases based on hybrid silica supports, the diversity or similarity of phases provided by one manufacturer, and the impact of evolutions in phase designs are readily apparent using this approach.
References
(1) E. Lesellier, LCGC North Am.34(10), 66–77 (2016).
(2) E. Lesellier, and C. West, J. Chromatogr. A1158, 329–360 (2007).
(3) E. Lesellier, J. Sep. Sci.33, 3097–3105 (2010).
(4) E. Lesellier, J. Chromatogr. A 1266, 34–42 (2012).
(5) J.W. Coym, J. Sep. Sci. 31, 1712–1718 (2008).
(6) E. Lesellier, C. West, and A. Tchapla J. Chromatogr. A1111, 62–70 (2006).
(7) E. Lesellier, and C. West, J. Chromatogr. A1149, 345–357 (2007).
Eric Lesellier is the guest author of this installment. David S. Bell is the editor of Column Watch.

Eric Lesellier has been an Associate Professor at the University of Paris Saclay (Orsay) since 1990, while carrying out his research at the Institute of Organic and Analytical Chemical (ICOA, CNRS UMR 7311), in Orléans, France. He is mainly interested in the understanding and modeling (LSER) of molecular interactions in supercritical fluid chromatography, and classification (map) systems (carotenoid test; spider diagram), of both stationary phases and solvent properties used for separation and extraction.
David S. Bell is a Senior R&D Manager at MilliporeSigma (formerly Sigma-Aldrich/Supelco). With a B.S. degree from SUNY Plattsburgh and a PhD in Analytical

Chemistry from The Pennsylvania State University, Dave spent the first decade of his career within the pharmaceutical industry performing analytical method development using various forms of chromatography and electrophoresis. During the past 15 years, working directly in the chromatography industry, Dave has focused his efforts on the design, development, and application of stationary phases for use in HPLC and hyphenated techniques. In his current role at MilliporeSigma, Dr. Bell’s main focus has been to research, publish, and present on the topic of molecular interactions that contribute to retention and selectivity in an array of chromatographic processes. Direct correspondence to: LCGCedit@ubm.com

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.














