Addressing the Challenges of Drug-to-Antibody Measurement
Antibody–drug conjugates (ADC) are an emerging pharmaceutical technology, with the potential of being the true “magic bullet”. However, these inherently complex biomolecules have unique analytical challenges. One is the determination of the drug-to-antibody ratio (DAR). The average DAR and the drug distribution need to be monitored and could determine the success of an ADC. This article discusses the chromatographic methods used to determine DAR, including hydrophobic interaction chromatography (HIC), reversed phase, and liquid chromatography–mass spectrometry (LC–MS).
Photo Credit: Vchal/Shutterstock.com

Brian Rivera, Phenomenex, Torrance, California, USA
Antibody–drug conjugates (ADC) are an emerging pharmaceutical technology, with the potential of being the true “magic bullet”. However, these inherently complex biomolecules have unique analytical challenges. One is the determination of the drug-to-antibody ratio (DAR). The average DAR and the drug distribution need to be monitored and could determine the success of an ADC. This article discusses the chromatographic methods used to determine DAR, including hydrophobic interaction chromatography (HIC), reversed phase, and liquid chromatography–mass spectrometry (LC–MS).
Antibody–drug conjugates, or ADCs, are monoclonal antibodies coupled to a small molecule, typically a highly potent cytotoxic agent. The main components of an ADC are the antibody, a linker, and the small molecule, typically called the warhead or payload. ADCs utilize the binding specificity of a monoclonal antibody to deliver the payload to a target cell. For example, a cytotoxic agent like a tubulin or mitotic inhibitor can be conjugated to a wellâcharacterized monoclonal antibody that is specific to a known receptor expressed by cancerous cells. Once the ADC binds to the target cell, it is internalized via receptorâmediated endocytosis. The linker is then cleaved and the cell undergoes apoptosis. Because ADCs are site-directed and specific, they can be up to one thousand times more potent than chemotherapy (1). This marriage of small and large molecule has been hailed as the “magic bullet”, far more effective than Nobel Laureate Paul Ehlrich could have ever imagined (2).
However, along with the potential and promise of ADCs comes their inherent complexity. Characterization of a 150 kD, nearly 1300-amino-acid protein is already difficult. Conjugation of the mAb introduces heterogeneity that is further complicated with multiple linker chemistries and various cytotoxic payloads. Figure 1 illustrates the conjugation sites for the payload and heterogeneity of a typical cysteine conjugate. The analytical challenge then comes in quantitating and distinguishing these different ADC species.
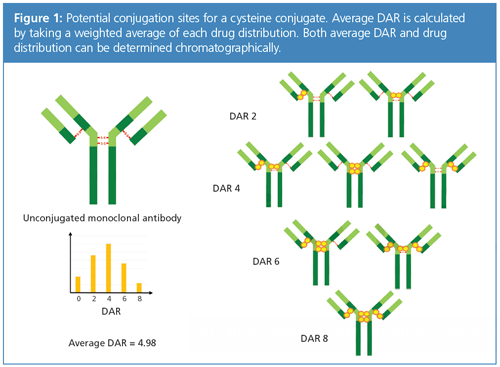
Nonetheless, characterization must be done, and one critical quality attribute of the ADC that needs to be monitored throughout all stages of development and manufacture is the average number of drugs conjugated to the mAb. This is referred to as the drugâto-antibody ratio (DAR) and not only is it indicative of drug efficacy but also safety (3). In addition, lower DAR gives a wider therapeutic window, while higher DAR is associated with toxicity and immunogenicity (4). DAR can be expressed as a weighted average (DAR average), essentially a molar ratio of drugs loaded per mAb. DAR can also describe drug distribution, which is an indicator of heterogeneity of the conjugate. For clarity, when DAR is mentioned in this article, it will be average DAR, unless otherwise specified.
DAR can be determined using a variety of techniques, the simplest of which is ultraviolet–visible (UV–vis) spectroscopy, essentially comparing the absorbance max. of the payload and the antibody (that is, 280 nm). However, this must be qualified with confirmed orthogonal techniques, typically liquid chromatography (LC) or high-resolution accurate mass spectrometry (HRAM). This article will cover current LC methods for determination of DAR and their challenges and limitations in the expanding field of ADCs.
HIC for ADCs
Hydrophobic interaction chromatography, or HIC, is the most commonly used method to determine DAR and drug distribution for ADCs. Although HIC relies on hydrophobic interactions much like reversed phase chromatography, some context is needed for macromolecules. To remain in a thermodynamically favourable state, hydrophobic regions of proteins self-associate in an aqueous environment. Introducing so-called lyotropic salts increases solvent surface tension, thus increasing the solubility of the buried hydrophobic regions of a protein. By increasing salts in the mobile phase, the macromolecule can then weakly bind to hydrophobic stationary phases. As the amount of salt decreases, the interaction between hydrophobic regions lessens as the protein adopts a more favourable thermodynamic state. Thus, HIC uses a descending salt gradient and separates analytes in order of hydrophobicity (5).
During its initial adoption, HIC was primarily used as a purification method because it is not denaturing like reversed phase (6). As such, it can be used when the function (that is, the tertiary and quaternary structure) of the protein needs to be maintained. Another distinguishing factor between HIC and reversed phase is the use of relatively low hydrophobicity alkyl- and aryl-phases with very low bonded densities. In preparative, low-pressure chromatography, cross-linked dextrans or agarose are functionalized with butyl and phenyl phases. For analytical methods, HIC was initially used to assess protein misfolding or for identification of variants as an orthogonal method to reversed phase (7,8). Here, nonâporous, hydrophilic coatedâpolystyrene or polymethacrylate particles are functionalized with the same low-bonded density, low hydrophobicity ligands used in preparative chromatography. However, with the emergence of ADCs and the use of HIC for DAR and drug distribution, column manufacturers began to offer more HIC phases to address the constantly evolving chemistry of linkers and payloads. In addition to butyl and phenyl selectivities, other short alkyl chains (for example, ethyl and propyl), alkylamide, and ether phases are available. Sub-3-µm and even sub-2-µm particles are available, in line with the shift to ultrahighâpressure liquid chromatography (UHPLC) for large molecules for faster methods and better resolution. That said, 4.6- or 4.0-mm internal diameters are still the most commonly used dimensions for DAR work.
One of the most popular HIC columns for DAR is the nonporous polymethacrylate butyl phase (9). Again, peaks are eluted in order of hydrophobicity; for ADCs, a mAb with zero drug load will elute first, with each subsequent drug load eluting later. Average DAR is calculated using weighted peak areas for the different DAR species. As such, it becomes important to ensure the method is capable of baseline separation of the different drug loads. In addition, a good separation can distinguish different payload positional isomers. That is, different species of DAR-4, depending on the conjugation site, might be separated as long as gradient steepness is optimized and phase selection is appropriate. Although not a method imperative per se, it might be helpful to provide further information of the conjugate.
As mentioned previously, UV–vis is often used to calculate DAR, and this can be combined with HIC to confirm DAR species. Both payload and antibody have distinctive absorbance maxima. If a diode array detector is used, both wavelengths can be monitored during the high performance liquid chromatography (HPLC) run to identify and confirm peaks. Figure 2 shows a HIC profile and absorbance spectra increasing at 252 nm absorbance as drug load increases.
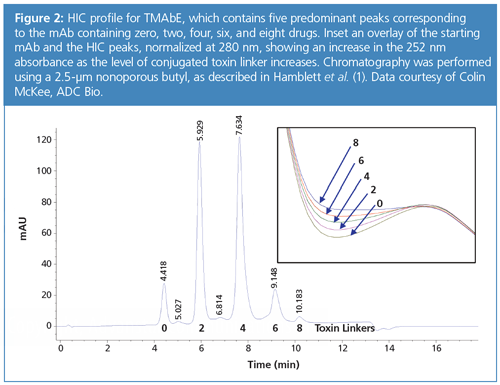
Finally, for cysteine conjugates, which have a maximum of eight drug loads for IgG1 mAbs, typically DAR-6 and DAR-8 drug loads will not elute with conventional linker and payload chemistries. However, optimization of temperature and the addition of organic modifier can help not only with elution but also with selectivity for higher drug loads (10).
Limitations of HIC
Although it is nondenaturing and can give the most quantitative data for ADCs, HIC is inherently a low-resolution technique. HIC uses lyotropic salts to modulate retention, so selectivity tends to be low. Other limitations as a separation technique are that any post-translational modification unrelated to the payload may result in discrepancies in DAR values. Either way, relative to reversed phase, which uses nonpolar solvents that can distinguish very small differences in hydrophobicity, HIC is low in resolution.
This is important considering the differences in hydrophobicity of ADC species with higher drug loads. For example, an ADC with DAR-8 may be too hydrophobic to separate by HIC and separation of the higher DAR species becomes exceedingly difficult. This is because the hydrophobicity of DAR-6 and DAR-8 is very similar. As demonstrated by Bobaly et al., a concave gradient might be necessary to optimize resolution for the higher drug loads (11). A logarithmic gradient profile implemented in this study successfully separated DAR-6 and -8 species of a cysteine conjugate.
HIC has only been demonstrated on a limited number of ADC chemistries, for example, the FDA-approved Adcetris, which is a cysteine conjugate (12). The nonporous butyl or ether columns have selectivity to separate the different DAR species of a cysteine conjugate quite well. As a result of their inherent heterogeneity, lysine conjugates typically cannot be separated. However, with the growing number of different ADC platforms, from engineered cysteines to glycan conjugates, commercially available HIC chemistries may not provide sufficient separation. Further, more hydrophilic conjugates-both with hydrophilic linkers and hydrophilic payloads-are being developed. Lyon and colleagues utilized different linker chemistries, deviating from the common “valine-citrulline-PABC” motif, to reduce hydrophobicity of an MMAE conjugate for improved pharmacokinetics (13). Higher drug load species using traditional hydrophobic linkers could not elute from the columns used in this paper. Other charged or hydrophilic linkers are being utilized in so-called third generation ADCs that are being evaluated to reduce multi-drug resistance (14). As such, the use of a hydrophilic linker combined with higher DAR species may lead to conjugates that do not separate using currently available HIC chemistries. This becomes even more apparent as more polar payloads are being used. Antibody-coupled siRNA conjugates are a special instance where the warhead is a silencing RNA (15). Because of the polar nature of nucleic acids, HIC has little utility in determining drug load.
Alternatives to HIC
HIC is the primary method for drug distribution and average DAR, but there are orthogonal LC methods that can be used for confirmation. Again, although related, reversed phase has superior resolution. There are some limitations to direct analysis of ADCs, in particular, cysteine conjugates are held together by noncovalent hydrophobic interactions of the payload and these will be disrupted using the denaturing conditions of reversed phase conditions (16). However, reduction of the ADC can be performed and analyzed by reversed phase for average DAR and DAR distribution (17). Instead of separation of individual, intact DAR species, weighted average of peak areas for conjugated and unconjugated antibody fragments (that is, dissociated heavy and light chains) is summed, then doubled to calculate the intact drug load. This strategy can also be used for site-specific conjugates, although in this instance, intact ADC may be an acceptable method. Xu and colleagues showed separation of DAR reduced and intact reversed phase with comparable results (18).
Finally, it is important to mention LC–MS for determining DAR and its distribution. Using intact LC–MS methods with wide-pore columns, DAR can quickly be determined, provided a high-resolution MS like a QTOF or Orbitrap is used. However, because traditional LC–MS utilizes the same denaturing mobile phases as reversed phase, this intact reversed phase is limited to lysine and siteâspecific conjugates. Typically, deglycosylation is performed, and the intact protein is analyzed using a wide-pore silica or polymer-based column. DAR is then determined using deconvoluted mass spectrum. The masses are separated by a shift in mass-to-charge ratio (m/z) of linker and drug (for example, 958 Da for DM1 drug and a MCC linker), which corresponds to different drug loads (19). Another approach would be native-MS. With this method, a size-exclusion chromatography (SEC) desalting column is used, which allows for analysis of intact cysteine conjugates (20). The one caveat to either LC–MS approach is that there is a discrepancy between DAR values obtained by LC–MS because higher DAR species will have different ionization efficiencies (21). Thus, reported DAR will tend to be lower than HIC or even reversed phase methods. As such, even though MS might be the preferred route for rapid evaluation of conjugation quality, DAR values should be confirmed by one or more LC–UV-based methods.
Conclusions
HIC is the gold standard for determining DAR and drug distribution for ADCs. HIC is inherently a low-resolution technique, but there has been an increase in phase and particle technology to address the need for method development. Reversed phase can be used as an orthogonal separation for DAR, though this has its own unique challenges as well. Finally, MS can rapidly determine DAR, though there is still the need to confirm results with an LC–UV method that might be more quantitative. With the rapid pace of ADC development, there is a critical need for additional analytical tools to meet the challenges of DAR determination.
Acknowledgements
The author would like to acknowledge Szabolcs Fekete, Davy Guillarme, Srinivasa Rao, and Colin McKee for their thoughts and contributions to this article.
References
- E.L. Sievers, P.D. Senter, et al., Annu. Rev. Med.64, 15–29 (2013).
- N. Diamantis and U. Banerji, Br. J. Cancer 114(4), 362–7 (2016).
- B.A. Teicher and R.V.J. Chari, Clin. Cancer Res.17(20), 6389–6397 (2011).
- C. Peters and S. Brown, Biosci. Rep.35(4), 1–20 (2015).
- J. Valliere-douglass, A. Wallace, and A. Balland, J. Chromatogr. A1214(1–2), 81–9 (2008).
- J.T. Mccue, Meth. Enzymol.463, 405–14 (2009).
- A.J. Chirino and A. Mire-sluis, Nat. Biotechnol.22(11), 1383–91 (2004).
- C. Scheich, F.H. Niesen, R. Seckler, and K. Büssow, Protein Science: A Publication of the Protein Society13(2), 370–380 (2004).
- A. Cusumano, D. Guillarme, A. Beck, and S. Fekete, J. Pharm. Biomed. Anal.121, 161–73 (2016).
- K.J. Hamblett, P.D. Senter, D.F. Chace, M.M. Sun, J. Lenox, C.G. Cerveny, et al., Clin. Cancer Res. 10, 7063–70 (2004).
- B. Bobaly, A. Beck, J.L. Veuthey, D. Guillarme, and S. Fekete, J. Pharm. Biomed. Anal.131, 124–132 (2016).
- B. Bobály, G.M. Randazzo, S. Rudaz, D. Guillarme, and S. Fekete, J. Chromatogr. A1481, 82–91 (2017).
- Y.T. Adem, K.A. Schwarz, E. Duenas, T.W. Patapoff, W.J. Galush, and O. Esue, Bioconjug. Chem.25(4), 656–64 (2014).
- R.P. Lyon, T.D. Bovee, S.O. Doronina, et al., Nat. Biotechnol. 33(7), 733–5 (2015).
- A. Beck, L. Goetsch, C. Dumontet, and N. Corvaïa, Nat. Rev. Drug Discov. (2017).
- N. Bäumer, N. Appel, L. Terheyden, et al., Nat Protoc.11(1), 22–36 (2016).
- J. Ouyang, Methods Mol. Biol. 1045, 275–83 (2013).
- M.M. Sun, K.S. Beam, C.G. Cerveny, et al., Bioconjug. Chem.16(5), 1282–90 (2005).
- Y. Xu, J. Guifeng, T. Cuong, L. Xiaofan, et al., Process Research & Development20.6, 1034–043 (2016).
- M.T. Kim, Y. Chen, J. Marhoul, and F. Jacobson, Bioconjug. Chem. 25(7), 1223–32 (2014).
- J.F. Valliere-douglass, W.A. Mcfee, and O. Salas-solano, Anal. Chem.84(6), 2843–9 (2012).
- K.J. Pacholarz and P.E. Barran, EuPA Open Proteomics11, 23–27 (2016).
Brian Rivera is the Bioseparations Product Manager with Phenomenex. He has over 10 years of experience in protein HPLC method development, focusing mainly on size-exclusion chromatography and glycan mapping.
E-mail:brianr@phenomenex.comWebsite: www.phenomenex.com


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












