A Look at Improved Aroma Profiling of Foods by High-Capacity Sorptive Extraction
High-capacity sorptive extraction combined with thermal desorption–gas chromatography–mass spectrometry (TD–GC–MS) can improve on traditional methods for the analysis of VOCs and other trace analytes that contribute to the aroma, flavor, and safety of food products.
High-capacity sorptive extraction is a sampling technique that involves using a probe to extract volatile and semi-volatile organic compounds (VOCs and SVOCs) from samples for analysis by thermal desorption–gas chromatography–mass spectrometry (TD– GC–MS). Samples can be taken from the headspace of a liquid or a solid, or by immersing the probe in the liquid. This study demonstrates how the technique can improve on traditional methods in the analysis of VOCs that contribute to the aroma and flavor of a breakfast cereal and in identifying other compounds of interest, such as food additives, contaminants, and potentially toxic compounds at trace levels.
Within the food industry, there is an increasing need to monitor product quality and safety to understand flavor composition and product contamination, and to determine how these relate to both consumer satisfaction and effects on human health. This analysis requires a detailed understanding of individual components of a product, but conventional approaches to sample preparation and gas chromatography–mass spectrometry (GC–MS) analysis can struggle to provide the necessary level of sensitivity.
Traditionally, solid-phase microextraction (SPME)—in which a fiber coated with an extracting phase (known as the sorptive phase)—has been used to sample volatile organic compounds (VOCs) from foods and beverages. SPME offers simple, solvent- less sampling, and is easily automated for high-throughput applications. However, it is limited by the amount of sorptive phase (to ~0.5 μL) and the fragility of the fiber (1). More recently, thicker sorptive films and stir bars with a sorptive phase have been used to enhance sensitivity; however, the latter approach is limited in scope, due to the manual interactions required by the user (2).
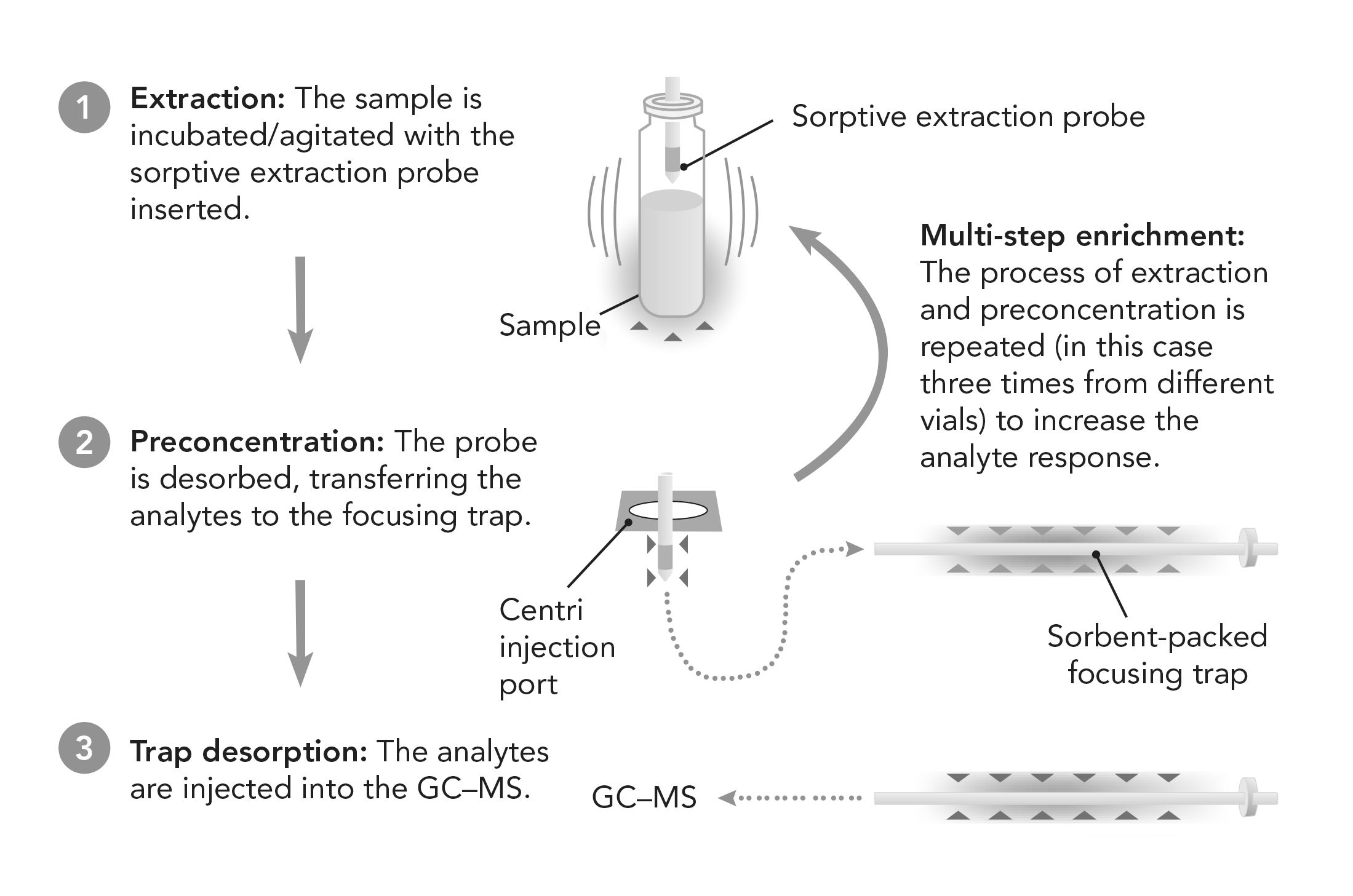
High-capacity sorptive extraction can overcome these issues. Firstly, the use of strong, metal-core probes supporting a higher amount of sorptive phase (~65 μL) means they are less prone to damage and provide a more robust extraction technique with improved sensitivity (3). The technique can also be fully automated, from sample extraction and post-sampling probe washing and drying through to desorption and GC injection. It is also possible to preconcentrate multiple extractions onto a single focusing trap prior to GC analysis, a process known as multi-step enrichment (MSE) (Figure 1). This increases the extraction efficiency of the technique, which leads to a higher column loading of analytes, enhancing the sensitivity and number of compounds detected.
FIGURE 1: High-capacity sorptive extraction with multi-step enrichment workflow.
In this study, the volatile organic chemical profile of a breakfast cereal was analyzed using high-capacity sorptive extraction first, and then combining it with MSE. This enabled a broad range of VOCs contributing to the flavor and aroma of the product to be identified. Additionally, food contaminants, potentially from the product’s packaging and the original cereal crop, were detected.
Experimental
Sample Preparation and Extraction
A store-bought breakfast cereal (1 g) was homogenized prior to addition of NaCl (2.5 g) and distilled water (10 mL). Headspace extraction of VOCs was performed on the Centri extraction and enrichment platform (Markes International Ltd.) using two modes: (a) HiSorb, a single-vial extraction for analysis and (b) HiSorb with MSE, loading four extractions from different, replicate sample vials to the trap prior to analysis. HiSorb probe: Short-length (48 mm) stainless steel (Markes International Ltd., part no. H1-XXABC); Incubation: 75 °C (20 min); Probe desorption: 250 °C (10 min); Flow path: 180 °C; Focusing trap: Materials Emissions (U-T12ME-2S) (Markes International Ltd.); Purge flow: 50 mL/min (1 min); Trap low: 20 °C; Trap high: 280 °C (5 min); Heating rate: MAX (>100 °C/s); Split flow: 5 mL/min.
Gas Chromatography (GC): Instrument
Agilent 7890 GC system (Agilent Technologies); Column type: TG-624, 30 m × 0.25 mm × 1.4-μm (Thermo Fisher); Column flow: Helium, 1.5 mL/min (constant flow); Oven program: 50 °C (5 min), 15 °C/min to 240 °C (10 min).
Mass Spectrometry (MS)
Instrument: BenchTOF-Select mass spectrometer (SepSolve Analytical); Mass range: m/z 33–300; Acquisition rate: 8 Hz.
ChromSpace GC×GC software(SepSolve Analytical) was used for full instrument control and data processing.
Results and Discussion
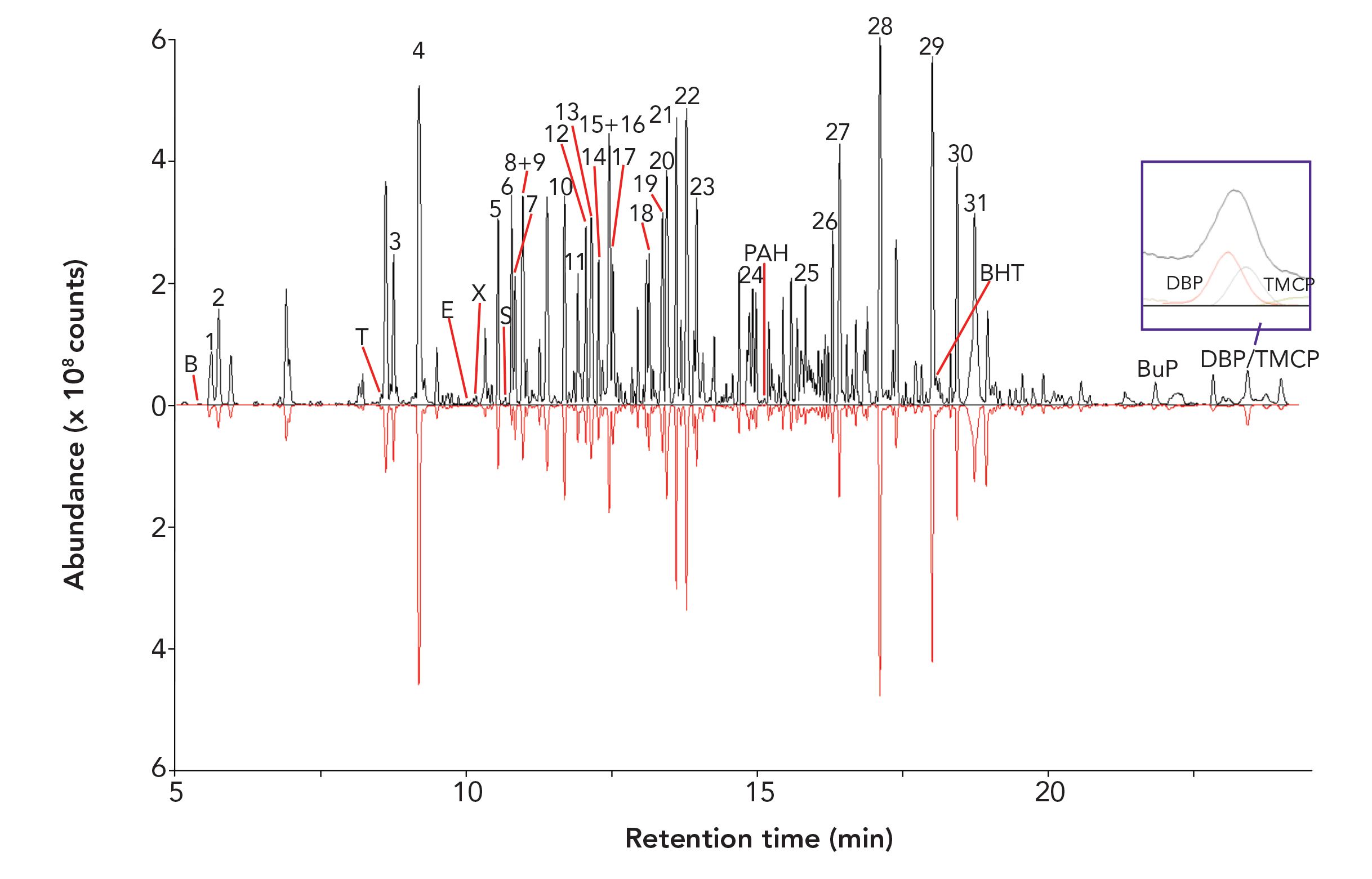
Volatile and Semi-Volatile Organic Compounds (VOC/SVOC) Profile Analysis Cereal samples were extracted using sorptive extraction probes, initially for a single-vial analysis, followed by MSE, where four headspace extractions were taken from separate vials for one GC analysis. Figure 2 shows the total ion chromatogram (TIC) for both the enriched (upper black trace) and single (lower inverted red trace) extractions. It is clear that an enhancement in sensitivity is achieved across the entire sample profile when using enrichment compared to a single extraction, with no compromise in peak symmetry or resolution. MSE provided a more comprehensive sample profile so the results using this technique are discussed in the following sections.
FIGURE 2: Headspace TIC profile comparison of the breakfast cereal – single extraction (lower inverted red trace) and MSE (4× extractions, upper black trace). Inset shows deconvolved elution profiles typically used for identification, in this instance showing the contaminants dibutyl phthalate (DBP) and tris(2-chloroisopropyl)phosphate (Amgard TMCP).
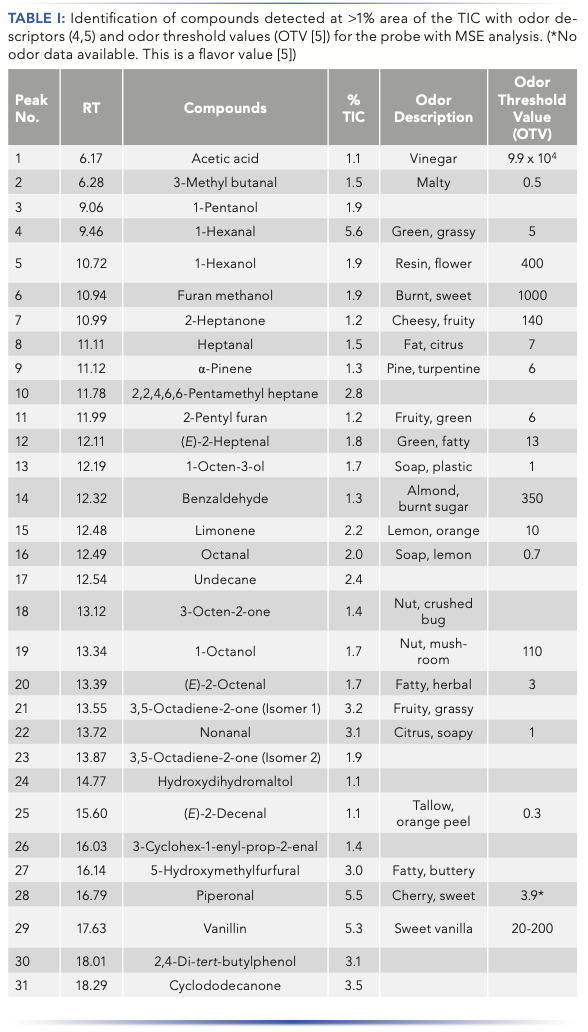
In total, 150 VOC/SVOC compounds were detected by MSE. The identification of compounds that had a peak area >1% of the TIC and with a NIST match factor >750 are shown in Table I, with odor descriptors (where available) and odor threshold values (the concentration at which an aroma or taste can be detected).
In a sample this complex with significant chromatographic co-elutions (where two or more compounds do not separate chromatographically so they are difficult to see), spectral deconvolution is critical for a more confident identification of VOCs present. Deconvolution is a data processing technique that can be applied to complex elution profiles. It was carried out in this study using data analysis software with resulting spectra compared to compounds in the NIST 2017/ EPA/NIH mass spectral library (6). The inset in Figure 2 shows an example of this. In this instance, the deconvolved peaks were identified as the SVOCs dibutyl phthalate (DBP, a plasticizer) and tris(2-chloroisopropyl) phosphate (Amgard TMCP, a flame retardant).
Other compounds of interest from a food toxicity or contamination perspective (text labelling in Figure 2) were also detected, and will be discussed in more detail later.
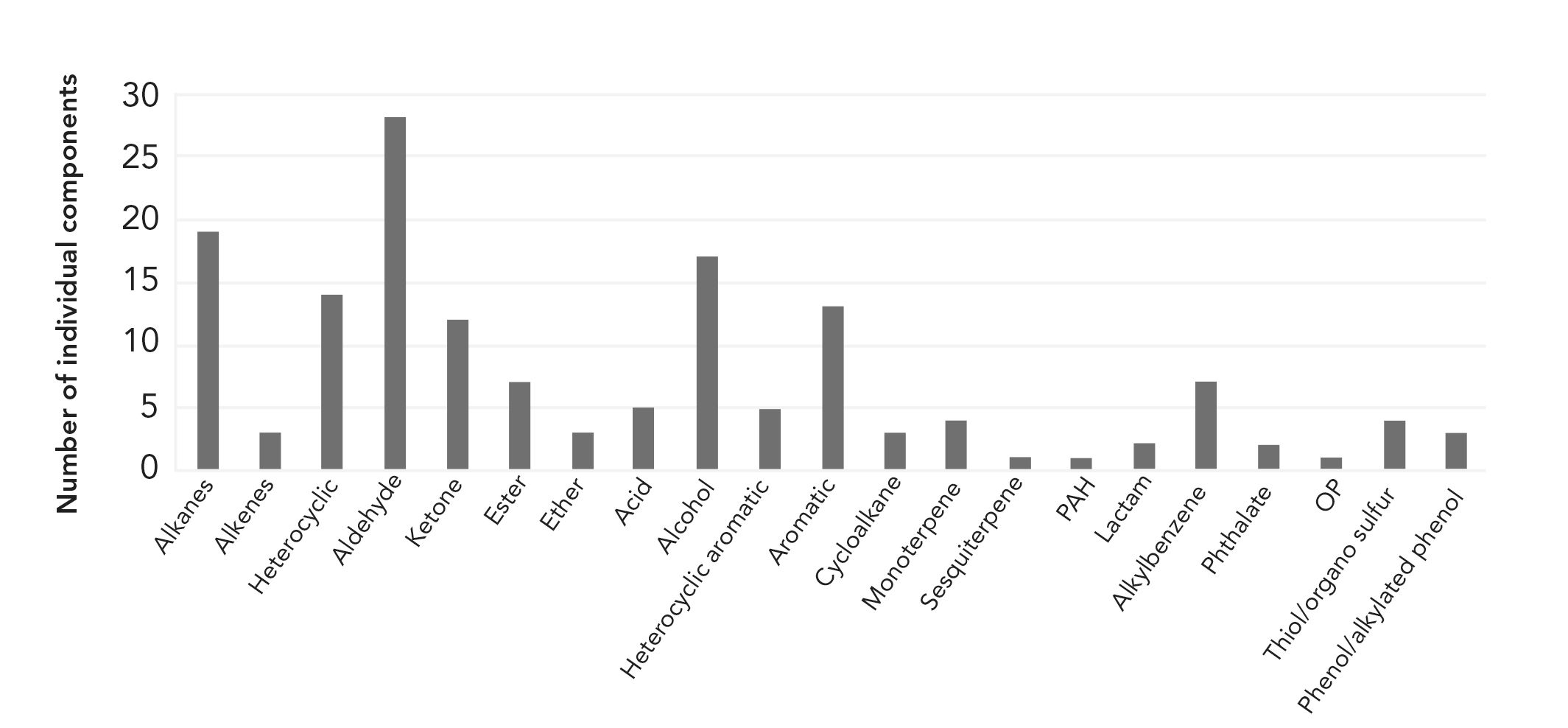
The range of VOC/SVOC compounds identified is wide, as demonstrated in Figure 3. The bar chart shows the distribution of the number of components for each compound class. The profile was dominated by aldehydes, alkanes, alcohols, heterocyclics, and aromatics. Within these classes, the most abundant compounds were hexanal (green, grassy) at 5.6% of the TIC, piperonal (cherry, sweet) at 5.5% of the TIC, and vanillin (sweet vanilla) at 5.3% of the TIC. During the production (heating) process of the cereal, Maillard reactions (chemical reactions between amino acids and reducing sugars) occur, and therefore a large amount of these compounds would be expected. Several compounds that are known to give an unpleasant or off odor were also detected, for example, four thiols or organo-sulfurs and three phenols (Figure 3), albeit at relatively low levels.
FIGURE 3: Number of components per compound class making up the VOC/SVOC profile of the cereal sample extracted by sorptive extraction probe with MSE.
Trace-Level Compound Identification
Principal component analyses for VOCs and SVOCs are important for characterizing the aroma and flavor profiles of food products, and the odor threshold value (OTV) is an associated parameter. Therefore, detection and identification of trace VOCs can be significant, particularly for compounds with medium and low OTVs, or for problematic compounds (such as malodors) with low values, so that subsequent remedial action can be taken if necessary.
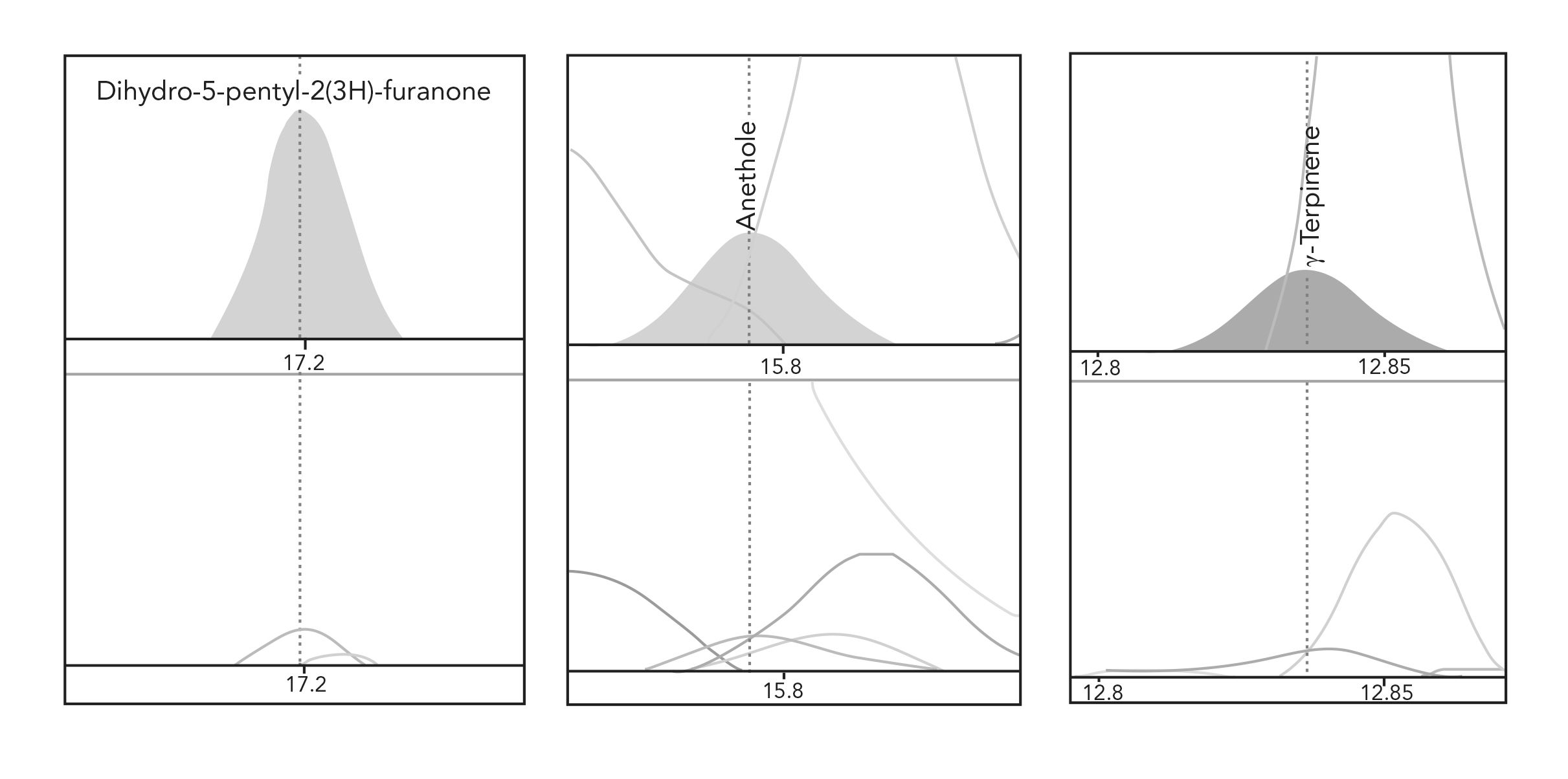
MSE provides a mechanism to increase the amount of each compound introduced to the GC, thus increasing the number of compounds detected, extending down to trace-level components. As representative examples, compounds such as dihydro-5-pentyl-2(3H)-furanone (γ-nonalactone), anethole, and γ-terpinene were detected in the single extraction but at very low levels where their spectral quality was not sufficient for a good library match. Figure 4 demonstrates how MSE enabled a more confident identification for these compounds, and this is indicated in the NIST values shown in parentheses in Table II.
FIGURE 4: Example peak profiles for compounds with minimal response and poor spectra from a single extraction (lower traces) compared to MSE (upper traces). Time (x-axis) vs. Abundance (y-axis).
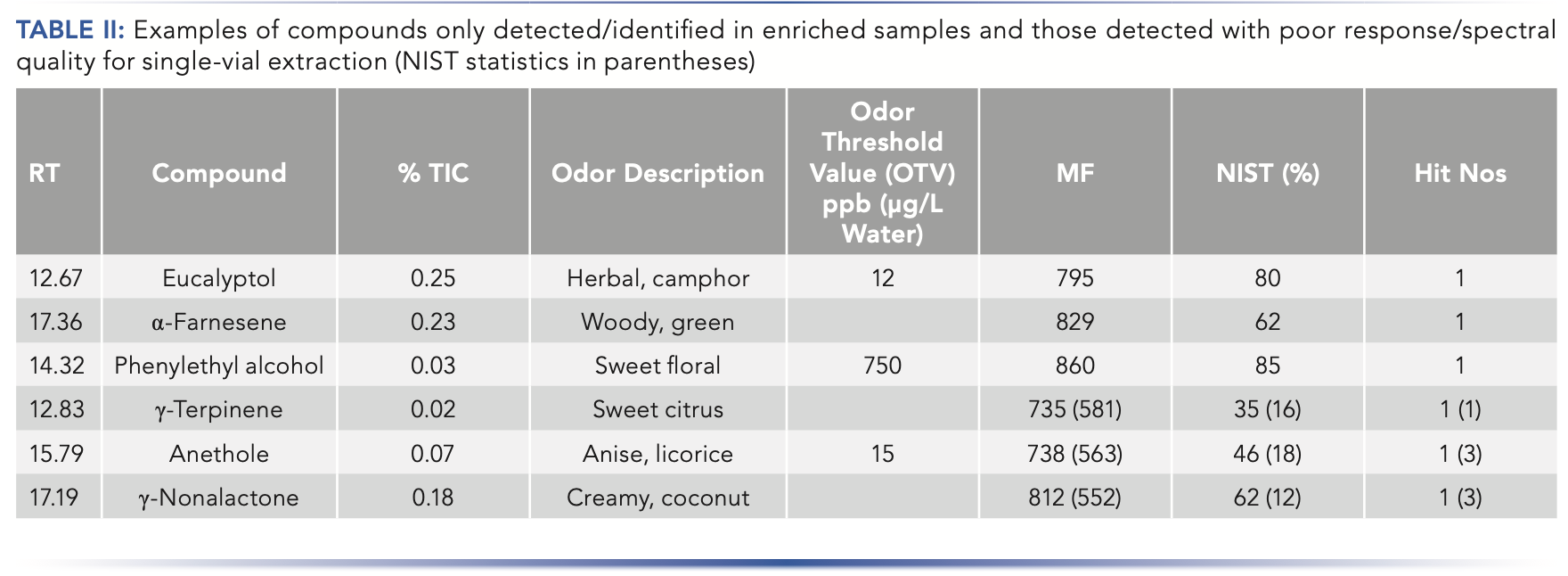
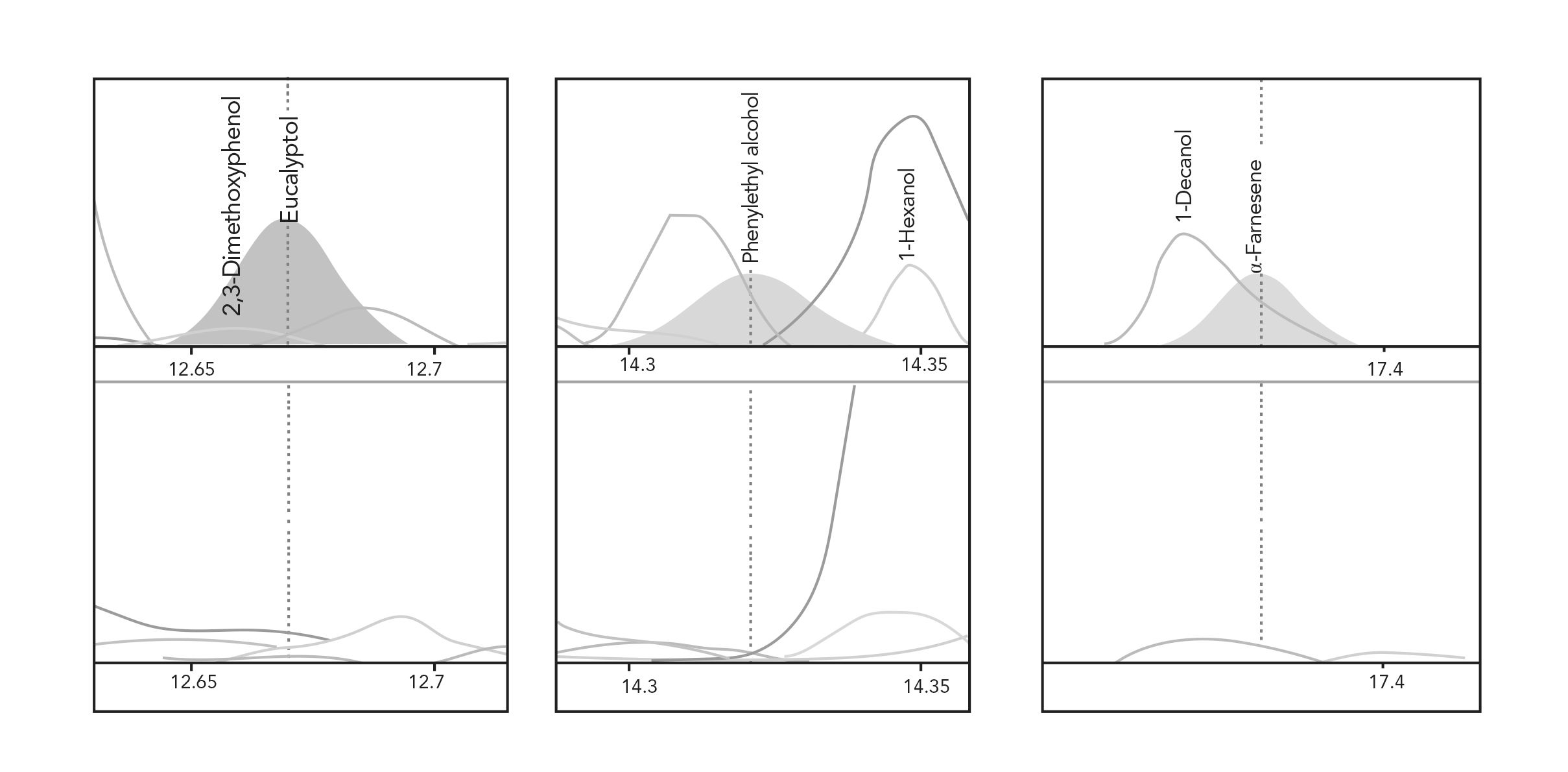
Other components, such as eucalyptol, phenyl ethyl alcohol, and α-farnesene were only detected in the enriched sample, meaning they could have been missed had only a single extraction been used for analysis (Figure 5). Eucalyptol is an important component in the sample with a relatively low OTV (12 ppb), imparting a herbal or camphor aroma to the product. These compounds are listed in Table II. The NIST values represent the enriched sample with comparison to the single point extraction in parenthesis when a partial response was observed.
FIGURE 5: Example peak profiles for compounds only detected using MSE (upper traces) compared to a single extraction (lower traces). Time (x-axis) vs. Abundance (y-axis).
Additives and Compounds with Potential Toxicity
Along with VOCs related to odor and taste, several additional compounds were identified in the breakfast cereal (Figure 2, Table III). These include the antioxidants butylated hydroxytoluene and 2,6-di-tert-butylphenol, known to be added to food to prevent spoilage, and several compounds that potentially originate from the food packaging as contamination: styrene, two phthalates, and two cyclic lactams. Interestingly, compounds that are usually associated with combustion were also detected in this sample (benzene, toluene, ethylbenzene, and xylene), with the possibility that these are present due to contamination of the original cereal crop.
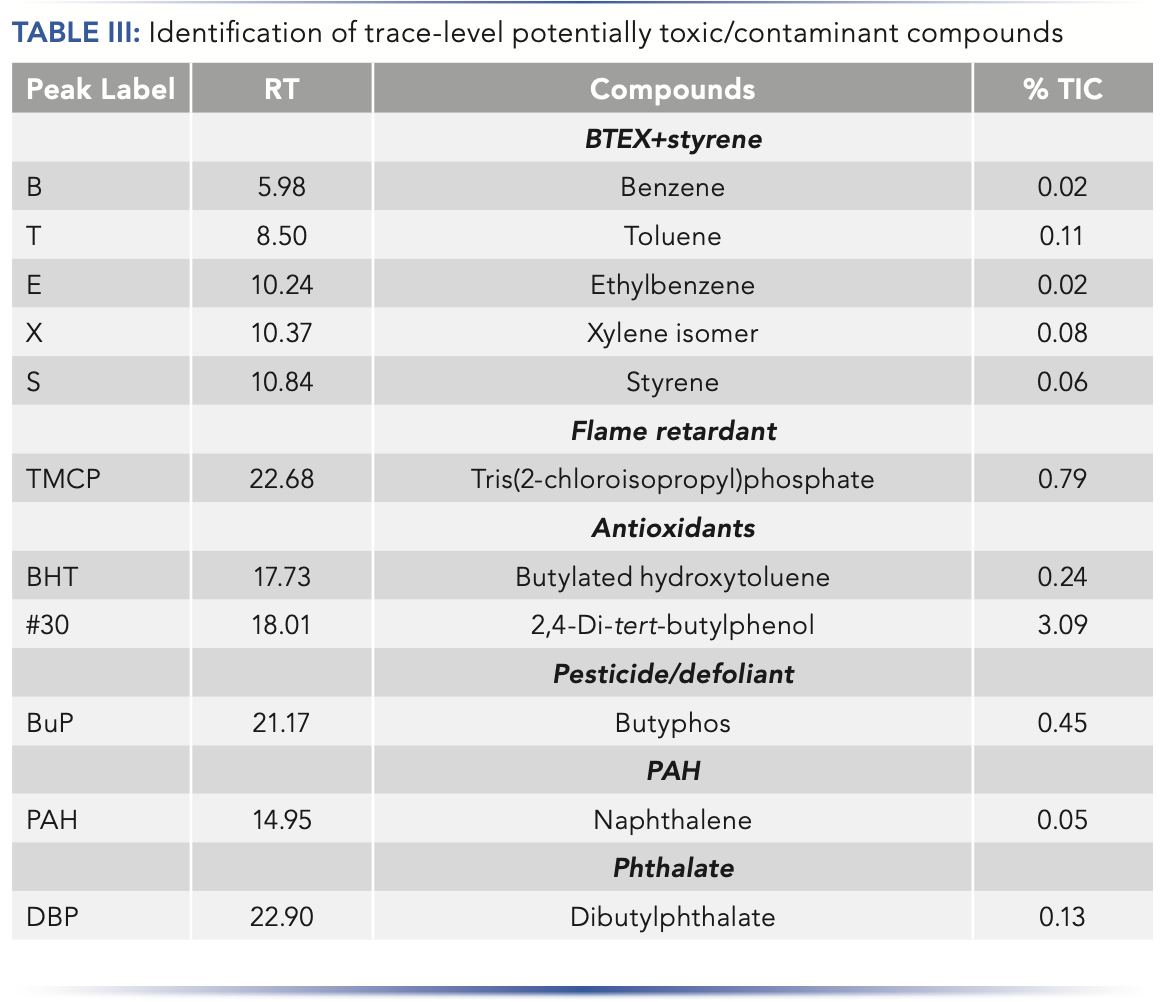
Furthermore, compounds present on the US EPA Toxic Substances Control Act (TSCA) list (7) were tentatively identified (Figure 2, Table III), such as butyphos (tributylphospine oxide), a herbicide, Amgard TMCP (tris(2-chloroisopropyl) phosphate), a flame retardant, and naphthalene, a PAH.
Although this analysis was only qualitative, so no indication of the concentration can be inferred from the data, the detection and tentative identification of these compounds demonstrates that high-capacity sorptive extraction can provide the ability to extract and analyze a wide range of compounds in addition to the main flavor and odor-active compounds of interest.
Conclusions
In this study, we have demonstrated the use of a fully automated high-capacity sorptive extraction technique for profiling the VOCs present in the headspace of a breakfast cereal using preconcentration with a single-vial extraction and multiple extractions. A wide range of compounds pertaining to taste and odor were found. Several components were found only in the enriched sample, demonstrating how multi-step enrichment provides a more comprehensive extraction, which leads to a more accurate VOC profile of a food sample. The fast MS acquisition combined with data processing further enhanced the identification of compounds, enabling co-eluted peaks in the complex profiles to be distinguished into individual analyte peaks. Moreover, the ability to discover compounds present in the sample that either contribute to off odors, have migrated from the packaging, or are contaminants from the original food commodity is important because they have potential toxicity and could affect human health. This breadth of compounds discovered demonstrates the enhanced capability of the extraction methodology. Lastly, the automated extraction and enrichment process means that no human interaction is required from sample extraction through to GC injection, allowing faster sample throughput. This ensures high productivity is achieved in industrial laboratories, such as contract service or food research.
References
(1) N.-E. Song, J.-Y. Lee, Y.-Y. Lee, J.-D. Park, and H.W. Jang, Appl. Biol. Chem. 62(16), (2019).
(2) U. Telgheder, N. Bader, and N. Alshelmani, Asian J. Nanosci. Mater. 1(2), 56–62 (2018).
(3) https://www.markes.com/Products/ Sampling-accessories/HiSorb-sorptive-extraction.aspx
(4) The Good Scents Company Information System, www.thegoodscentscompany.com, search facility accessed on Sep. 11, 2020.
(5) Odor and Flavor Detection Thresholds in Water (ppb) – Leffingwell & Associates, http://www.leffingwell.com/odorthre.htm, accessed on Sep. 11, 2020.
(6) https://sciencesolutions.wiley. com/solutions/technique/lc-ms/nist-epa-nih-ms-ms-mass-spectral-library-2017/
(7) United States Environment Protection Agency, www.epa.gov/chemicals-under-tsca, accessed on Sep. 11, 2020.
About The Authors

Rachael Szafnauer received an M.Sci in forensic science from the University of South Wales, UK, where her final-year project focused on fingerprinting emerging psychoactive substances using advanced techniques such as GC×GC–TOF MS, in collaboration with Markes International. She later took up the role of Thermal Desorption Product Specialist at Markes, providing technical and application sup- port to the commercial team, before taking on her current role at the start of this year, specializing in the development of applications using extraction and enrichment techniques.

Elinor Hughes obtained her B.Sc. in chemistry and Ph.D. in organic chemistry at Bangor University, UK. After working for a chemical manufacturing company for three years, she moved to the Royal Society of Chemistry where she worked in journals publishing for six years and on Chemistry World magazine for four years. This was followed by five years as a freelance copyeditor and science writer. Her current role is Technical Copywriter at Markes International.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.