The Year of the Laser
LCGC Europe
The concluding part of the series on the role of system suitability tests in the pharmaceutical industry.
In mass spectrometry (MS) the laser, together with electrospray ionization (ESI), has become an essential tool to advance our analytical pursuits in the sciences. The 50th anniversary of its invention marks an appropriate time to examine the device's importance in MS practice.
History credits the Hughes Research Lab's Theodore Maiman as the first to successfully operate a laser, in 1960. Since then, according to the LaserFest website, the US Patent office has granted more than 55000 patents involving the laser.
The laser has become an important asset in mass spectrometry (MS). Indeed, its usefulness nearly rivals that of electrospray ionization (ESI) in the ability to examine the molecular underpinnings of biological systems. So it is fitting that we consider the laser from a perspective of MS practice, and speculate on its future.
The Optical Society of America (OSA) and the American Physical Society (APS) are jointly promoting LaserFest (www.laserfest.org), which they describe as "a multi-year celebration designed to commemorate the 50th anniversary of the invention of the laser." Toward that end, the partnering societies plan a range of activities including public outreach events, lectures, symposia and educational demonstrations. Many of the proceedings will recognize and honour the accomplishments of those who discovered, developed and applied laser technology. Among those memorialized will be Charles Townes, who figures prominently among the pioneers of laser technology. Townes developed the forerunner to the laser — the "maser" — and published, with Bell Laboratories' Arthur Schawlow, a key theoretical paper in 1958 that led to the laser's commercial development. In 1960, Townes and Schawlow were jointly awarded the first laser patent.
Laser Types
The four types of lasers are gas, solid, liquid, and semiconductor.
Low-cost, helium-neon (HeNe) lasers are ubiquitous in the educational setting and emit energy at a variety of wavelengths. The typical low-cost units operate at 633 nm and are common in education. The HeNe mixture — the gain medium — is pressurized so that helium = 1 Torr and neon = 0.1 Torr. The gas mixtures contained within a glass tube of 1 cm diameter and 0.25–1 m length. Two electrodes connect to a high-voltage DC source that generates a discharge inside the tube acting like a "pump". Two parallel mirrors are placed around the gain medium, one in front of the other, so that only mirror 1 shows the complete reflection. Mirror 2 shows a partial reflection. When electric current passes through, a continuous light wave flows inside the tube with constant frequency, developing "coherent" light waves that emanate from mirror 2.
MS systems often trigger ionization by means of a nitrogen laser. Others, though, like the solid-state diode YAG lasers (neodymium-doped ytterbium aluminum garnet), are also used. A chemical matrix protects the molecule of interest from destruction by direct laser energy and enables vaporization and ionization. Essentially a chromophore, the matrix absorbs laser energy, which, liberated from the surface (where both matrix and analyte have been applied), then transfers or assists ionization of the analyte.
Other examples of gas lasers include higher efficiency, carbon dioxide units that emit high-power beams at about 10 µm. Such devices are often are used in industry for cutting and welding. Argon-ion lasers emit light in the 351–528.7 nm range. Depending upon the associated optics and type of laser tube, a variable number of lines are usable. Nevertheless, the most frequently used lines are 458 nm, 488 nm and 514.5 nm. Metal-ion lasers such as helium-silver (HeAg, 224 nm) and neon-copper (NeCu, 248 nm) generate deep, ultraviolet wavelengths. The very narrow oscillation line-widths of such lasers make them useful for fluorescence-suppressed Raman spectroscopy.
In solid lasers, a ruby-like cylindrical crystal serves as the gain medium, which is surrounded by a helical xenon flash lamp that acts as a "pump". Mirrors are arranged similar to those of gas lasers. A red laser beam results on the discharge of electric current.
Liquid lasers use organic dyes as their gain medium. In semiconductor lasers, known as injection laser diodes (ILD), current causes visible light modulation from the ILD. These lasers are often used in electronic equipment.
MS Applications
Karas and Hillenkamp introduced matrix-assisted laser desorption ionization (MALDI) in 1985.1 Along with electrospray ionization, MALDI has dramatically affected the way we investigate biomolecules, particularly proteins. Initially used to analyse intact proteins, MALDI is now widely used to analyse peptides that result from protein digestion. (Enzymatic protein digestion, a standard procedure in proteomics, is a means for identifying proteins by reducing the larger molecule into significant subsets.) MALDI's production of singly charged ions, which simplifies spectral interpretation, is also a prominent advantage for synthetic polymer analysis.
MALDI is well represented at the annual American Society for Mass Spectrometry (ASMS) each year, where about 6000 attendees bring about 3000 talks and posters. In 2010, there were 499 focused on MALDI. The numbers were slightly larger in past years (748 in 2004 or 30% of all abstracts submitted to ASMS that year), but MALDI clearly remains a significant identifiable technique.
A "soft" ionization technique (relative to electron ionization, for instance), MALDI especially benefits analyses of biomolecules and biopolymers (proteins, peptides and sugars) and other large organic molecules that tend to fragment when desorbed and ionized by conventional ionization methods. MALDI is similar to ESI in that both are soft techniques that are less likely to damage a molecule of interest. Both ESI and MALDI conserve charge and display good sensitivity. Yet MALDI yields many fewer and less abundant multiply charged ions; and electrospray, considered the more quantitative of the two techniques, produces more fragmentation from the multiply charged ions when appropriate activation is supplied after the ionization step.2,3
Three of MALDI's shortcomings become evident when the technique is applied to intact proteins and protein complexes. Because of possible source and analyser combinations for ESI and MALDI sources, the peaks of singly charged ions produced by MALDI are broader than the multiply charged, equivalent ions produced by ESI. Most limiting for the protein analysis using high performance mass spectrometers equipped with powerful analysers, however, is the mass range limitations: singly charged protein ions are simply not detected. Fragmenting singly charged ions is also more problematic, limiting structure determination from enzymatically digested proteins (or small proteins within the mass range of the instrument). Broad peaks make accurate mass determination difficult, and important subsequent steps like database searching yield only limited success. MALDI's other shortcoming is that a practitioner must find a suitable sample preparation method (Figure 1). Although MALDI generally requires little, if any, exotic sample preparation, it is sometimes difficult to find a matrix with properties that yield ions from solution complexes. Often, a peak for an intact, specific complex is observable only for the first application onto a fresh sample spot. Though a topic of some debate, it seems that if the same area is irradiated again, only dissociation products or nonspecific clusters are observed.4–6
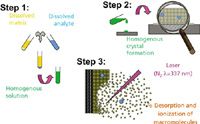
Figure 1: The matrix-assisted laser desorption ionization (MALDI) traditional solvent-based process as developed by Karas and Hillenkamp, with possible limitations at each step. (Courtesy S. Trimpin, Wayne State University.)
On the Horizon
Work by Trimpin and McEwen7 has shown that, contrary to conventional wisdom, using standard MALDI conditions can produce multiply charged ions similar to those obtained using ESI. A few references in the literature describe multiply charged ions similar to those created by ESI using a commercial, atmospheric pressure (AP-MALDI) infrared (IR) neodymium:yttrium aluminum garnet (Nd:YAG) laser to ablate an acidified glycerol matrix.8
David Muddiman's group (North Carolina State University, Raleigh, North Carolina, USA) developed a technique referred to as liquid matrix-assisted laser desorption electrospray ionization (liq-MALDESI), which generates multiply charged ions from liquid samples deposited onto a stainless steel sample target biased at a high potential. Muddiman says: "We have observed a singly charged radical cation of an electrochemically active species indicating oxidation occurs for analytes and therefore water; the latter would play a key role in the mechanism of ionization".9
Trimpin showed that UV nitrogen laser ablation of standard MALDI matrixes at atmosphere could produce multiply charged ions similar to ESI, with high sensitivity without an applied voltage. Calling the method laserspray ionization (LSI) distinguishes it from AP-MALDI, in which highly charged ions are not observable from solid matrices. In the initial work, the laser is shot through the sample holder in transmission mode (Figure 2). The matrix–analyte was placed in solution onto a glass microscope slide using the "dried droplet" method described by Karras10 in which the sample is allowed to dry and is then ablated using transmission geometry, in which the laser beam impinges the matrix from the bottom after passing through a glass microscope slide. Recent work shows the method can be applied using conventional reflective geometry on a commercial AP-MALDI source design as well.11 A major difference between LSI and other methods of producing multiply charged ions is that laser ablation occurs directly from a solid matrix and no voltage is required.

Figure 2: Laserspray ionization (LSI) schematic. In transmission geometry, laser ablation of the solid matrix/analyte sample occurs from the backside without the use of a voltage. In reflective geometry, the laser hits from the front. The transfer capillary, the region in which the matrix is removed from the highly charged matrix/analyte cluster, is crucial to produce ESI-like multiply charged LSI ions. (Courtesy S. Trimpin, Wayne State University.)
Forward momentum of charged matrix–analyte molten droplets is generated by the deposition of energy into the matrix by the laser pulse. The result propels the highly charged droplets sufficiently close to the MS ion entrance where they come under the influence of the vacuum and are transported with the flow. Just as in ESI, evaporation of the solvent (matrix) in the charged droplets is necessary to produce highly charged analyte ions.
According to McEwen, "The charge state can be switched to singly charged ions nearly instantaneously by changing the voltage applied to the MALDI target plate." Under normal, atmospheric MALDI operating conditions, a voltage is applied to the target plate, resulting in the observance of singly charged ions. The authors found that "At or near zero volts, highly charged ions are observed for peptides and proteins. Thus, switching between singly and multiply charged ions requires only the manipulation of a single voltage."
One of the great benefits of ESI as a technique — multiple charging that allows direct measurement of analytes well beyond the usual single-charge acquisition limits of the hardware — is thus achievable using the LSI source not achievable with AP-MALDI.
Practical Application of LSI
Trimpin focuses on resolving the shortcomings of MS laser applications: "Over the years, mass spectrometry has been a powerful analytical tool and a breakthrough in biological research. MS has been less successful in characterizing materials with restricted solubility and materials not easily ionized, whether due to complex composition or the desire to detect unadulterated or unmodified compounds."
When coupled with recent hybrid techniques like ion mobility, the LSI approach makes even more sense because it adds enhanced spatial resolution to the mix. Apart from its specific advantage of allowing a practitioner to choose between charge states (single versus multiple), LSI offers MALDI-like advantages: laser for high-spatial resolution, ability to work solvent-free for insoluble materials and speed. It also brings ESI-like advantages: working at atmospheric pressure, extended mass range of high performance mass spectrometers, and improved fragmentation (for example, electron-transfer dissociation, or ETD).7
In one experiment, a protein mixture of ubiquitin and lysozyme proteins in low abundance was used. Ion mobility data were obtained separately for both pure ubiquitin and lysozyme (Figure 3), to confirm plots of drift time versus m/z and associated multiply charged ion drifts. As Trimpin points out: "Without data extraction and time-intensive analyses, the pictorial snapshot readily determines the presence of both proteins." By selecting the specific part of the 2D plot for one protein, it is possible to extract m/z and drift-time information. In a similar case, β-amyloid isomers (1-42 and 42-1; MW 4511.5) were mobility separated. Both proteins displayed a similar multiple-charge envelope indistinguishable by a sole MS approach because of identical masses.

Figure 3: Low abundant mixture of proteins desorbed/ionized directly from a surface separated according to number of charges and cross-sections (defined by size and shape). The two-dimensional plot of drift time distribution (ion mobility dimension) versus m/z (time-of flight dimension) shows distinct multiple charge character and separation of the proteins. (Courtesy S. Trimpin, Wayne State University.)
As is the case with ESI, LSI produces singly charged ions from smaller analytes such as crude oil (Figure 4). Unlike ESI, singly charged LSI ions can be produced without using solvents; overcoming limitations and concerns of sample loss and the ability to ionize solubility restricted and ion suppressed parts of the sample composition.12

Figure 4: LSI Crude oil heat-assisted analysis using a common MALDI matrix 2,5-DHAP (2,5-dihydroxyacetophenone).7 (Courtesy S. Trimpin, Wayne State University.)
Spatial Imaging Approach for Synthetic and Biological Polymers
MALDI is useful for polymer analysis because of the spectral simplicity derived from singly charged ions (Figure 4). Trimpin, however, demonstrated that highly charged polymer ions as produced by ESI can be separated into charge state families using an ion mobility spectrometry (IMS)–MS approach. Now, the LSI approach can be used to produce ES- like polymer ions from the solid state. Polymer analysis by IMS–MS and the importance of producing multiply charged ions is shown on a branched four-arm PEG 800 consisting of an unknown number of isomeric structures [Figures 5(a) and (b)] and on an ubiquitin–polymer mixture [Figure 5(c)]. The branched four-arm PEG 800 doped with lithium acetate was analysed using ESI on a homebuilt 3 m long, high drift time resolution IMS-MS instrument [Figure 5(a)], not equipped with the heated desolvation device to perform LSI (Figure 2), and on a Synapt G2 (Waters Corporation, Milford, Massachusetts, USA) [Figures 5(b) and (c)]. The singly charged ions are not separated, however, for the doubly charged ions separation of isomeric structures are achieved with both instruments as is shown with the extracted drift time distributions for the ion with the m/z = 487. The charge repulsion, introduced with two cations present, causes structural changes of the isomers and the shape differences are used in the IMS dimension for separation. Figure 5(c) shows the drift time distribution versus m/z "snapshot" of a mixture of the four-arm PEG 800 doped with sodium chloride and the protein ubiquitin. From the snapshot of the mixture, low abundant features of +12 and +11 charge states of ubiquitin are evident. An inset of the 13 C isotope peaks for the +11 charge state can be used to determine the charge state and verify the molecular weight (8560 Da) on a mass range limited instrument (8000).

Figure 5: With multiply charged ions even isomeric compositions can be efficiently separated as depicted in (a) and (b), the IMSâMS display of drift time versus m/z; and (c) a 2-D "snapshot" of the polymers detecting small changes. (Courtesy S. Trimpin, Wayne State University.)
The DriftScope software (Waters Corporation) can be used to extract mass spectral and drift-time distribution data [Figures 5(a) and 5(b) inset] for detailed analysis. With multiply charged ions, even isomeric compositions can be separated efficiently and afford a 2-D "snapshot" of the polymers to detect small changes [Figure 5(c)] from sample to sample, which could be valuable for quality control and regulatory purposes.
The practical significance was highlighted in a review published by Weidner and Trimpin.11 They noted the literature on MS of polymers published from 2008 to 2009 (citations from SciFinder as of January 25, 2010, using restricted search terms "poly*" and "mass spectrometry") produced more than 750 relevant papers and five reviews. A current examination of techniques, theory and applications of ion mobility mass spectrometry is in press at this writing, edited by Wilkins and Trimpin.14
In other cases, we can see advantages using tools such as LSI to address a greater chemical diversity of analytical situations, for instance, the cellular components of the membrane forming the permeability barrier. The lipids in the plasma membrane are chiefly phospholipids, so as the plasma membrane faces watery solutions on both sides, its phospholipids form a bilayer in which the hydrophobic tails face each other. Under such conditions, intact analysis becomes more art than science.
That these practical findings come at a time when the skills of many operators have matured suggests the development of laser technology warrants our continued interest. Not only are nitrogen lasers inexpensive, but the ability to easily acquire accurate, narrow, multiply charged peaks along with intact mass from an easy-to-prepare sample is invaluable.
Sarah Trimpin is assistant professor at Wayne State University. She received her PhD at Max Planck Institute for Polymer Research, University of Mainz, Germany. My thanks to Ellen Inutan, a student at Wayne State, for her help in assembling the artwork.
"MS — The Practical Art" editor Michael P. Balogh is preincipal scientist at Waters Corp. (Milford Massachusetts, USA); a former adjunct professor and visiting scientist at Roger Williams University (Bristol, Rhode Island, USA); cofounder and current president of the Society for Smal Molecule Science (CoSMoS) and a member of LCGC Europe's editorial advisory board.
Direct correspondence about this column to LCGC Europe, Bridgegate Pavilion, Chester Business Park, Wrexham Road, Chester CH4 9QH, UK or e-mail: amatheson@advanstar.com
References
1. M. Karas, D. Bachmann and F. Hillenkamp, Anal. Chem., 57, 2935–2939 (1985).
2. J.M. Daniel et al., Int. J. Mass Spectrom., 216, 1–27 (2002).
3. V. Gabelica et al., J. Mass Spectrom., 38, 491–501 (2003).
4. B. Rosinke et al., J. Mass Spectrom., 30, 1462–1468 (1995).
5. M. Zehl and G. Allmaier, Anal. Chem., 77, 103–110 (2005).
6. S. Jespersen et al., J. Mass Spectrom., 33, 1088–1093 (1998).
7. S. Trimpin et al., Mol. Cell. Proteom., 9, 362–367 (2010).
8. S. Konig, O. Kollas and K. Dreisewerd, Anal. Chem., 79, 5484–5488 (2007).
9. J.S. Sampson, A.M. Hawkridge and D.C. Muddiman, Anal. Chem., 80(17), 6773–6778 (2008).
10. M. Karas and F. Hillenkamp, Anal. Chem., 60, 2299–2301 (1988).
11. C.N. McEwen, B.S. Larsen and S. Trimpin, Anal. Chem., 82, 4998–5001 (2010).
12. S. Trimpin, J. Mass Spectrom., 45(5), 471–485 (2010).
13. S.M. Weidner and S. Trimpin, Anal. Chem., 82, 4811–4829 (2010).
14. Ion Mobility Spectrometry: Theory and Applications, C. Wilkins and S. Trimpin, Eds. (CRC Press, Boca Raton, Florida, in press).

HPLC 2025 Preview: Fundamentally Speaking (Part 1)
May 13th 2025Michael Lämmerhofer from the Institute of Pharmaceutical Sciences, University of Tübingen, Germany, spoke to JFK Huber Lecture Award winner of 2024 Torgny Fornstedt, professor in analytical chemistry and leader of the Fundamental Separation Science Group, Karlstad University, Sweden, about his pioneering work in high performance liquid chromatography (HPLC) with a focus on fundamentals and industrial applications.
Determining Ways to Protect Honeybee Colonies with GC–MS
May 13th 2025A study conducted by the Agriculture Research Centre of Giza, Egypt, and Jilin Agricultural University in China, evaluated the efficacy of stinging nettle extract, nettle smoke, and formic acid in the controlling of Varroa mites, a major threat to honeybee colonies, with a focus on mite infestation reduction, honeybee mortality, and biochemical responses. Gas chromatography–mass spectrometry (GC–MS) was used to identify key bioactive compounds in the stinging nettle extract.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










