Why Use Miniaturized Columns in Liquid Chromatography? Benefits and Challenges
Reducing the column inner diameter results in a reduction in chromatographic dilution and increased sensitivity, allowing for more efficient tandem mass spectrometry sampling (MS/MS) and a higher number of molecule identifications.
While mass spectrometry coupled to liquid chromatography (LC–MS) has proven to be a powerful tool in the area of discovery omics (prote-, lipid-, metabol-, food-, and glycomics), omics research is often challenged by the complex nature of its samples. The identification and quantification of molecules of interest can prove particularly difficult when dealing with small amounts of samples and complex sample matrices (biofluids and single-cell analysis). Liquid chromatography column miniaturization can overcome these challenges. Reducing the column inner diameter results in a reduction in chromatographic dilution and increased sensitivity, allowing for more efficient tandem mass spectrometry sampling (MS/MS) and thus a higher number of molecule identifications.
Mass spectrometry coupled to liquid chromatography (LC–MS) has proven to be a powerful tool in discovery omics. MS advances in mass accuracy, sensitivity, resolving power, and scanning speeds over the last 20 years have made it possible to analyze molecules with high confidence and sensitivity (1,2). The coupling of a liquid chromatographic system to a mass spectrometer has allowed the profiling of biological samples by analyzing thousands of metabolites simultaneously. In proteomics, deep proteome coverage and post-translational modification (PTM) identification of phosphorylation, glycosylation, and other sites has been accomplished (3). In the world of foodomics, LC–MS allows for the crucial analysis of foods for nutritional content and contamination, which is of great concern to producers, governmental agencies, and the consumer (4).
Though there is no doubt that LC–MS is a powerful tool in discovery omics, it is often challenged by the complex nature of its samples. Metabolomics biomarkers tend to be present in extremely low amounts in biological matrices containing high concentrations of other compounds and contaminants, making these matrices extremely complex (5). Consequently, the search for metabolomic biomarkers can be hampered when using analytical-flow LC–MS (Table 1). Analytical LC–MS tends to favour the detection of metabolites already present at a high concentration; this is in part due to the difficult ionization that requires the use of high mass spectrometer source temperatures, voltages, and nebulization gases. Similarly, lipidomic analysis often uses nonvolatile salts, which exhibit poor desolvation and ionization efficiency (6,7). Ionization is not the only challenge. Sample amount can also be a dramatic limiting factor, such as in the case of biomedical and discovery proteomics that deal with low amount of samples, for example, single cells, biopsies, and tissues that do not provide the millions of cells or hundreds of micrograms of protein that are required in common proteomics workflows (1).
Liquid chromatography column miniaturization can overcome these challenges. Reducing the column inner diameter results in a reduction in chromatographic dilution. This dilution happens when a sample amount is injected into the column and it gets diluted by the surrounding solvents. In this case, less solvent means less chromatographic dilution, which is the result of using a smaller inner column diameter (Figure 1). A reduction in chromatographic dilution results in an increase in ion sensitivity, allowing for more efficient MS/MS sampling and thus a higher number of molecule identifications (2).
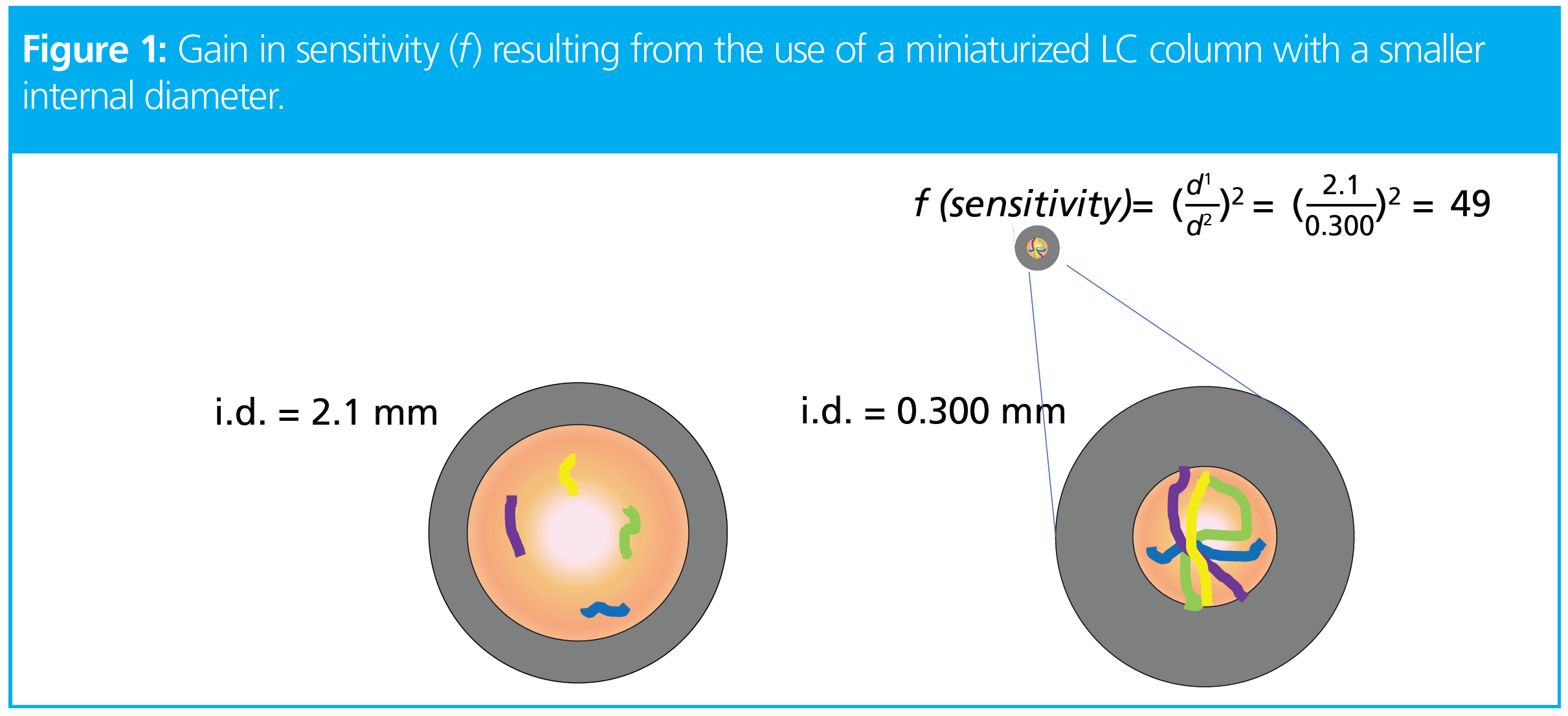
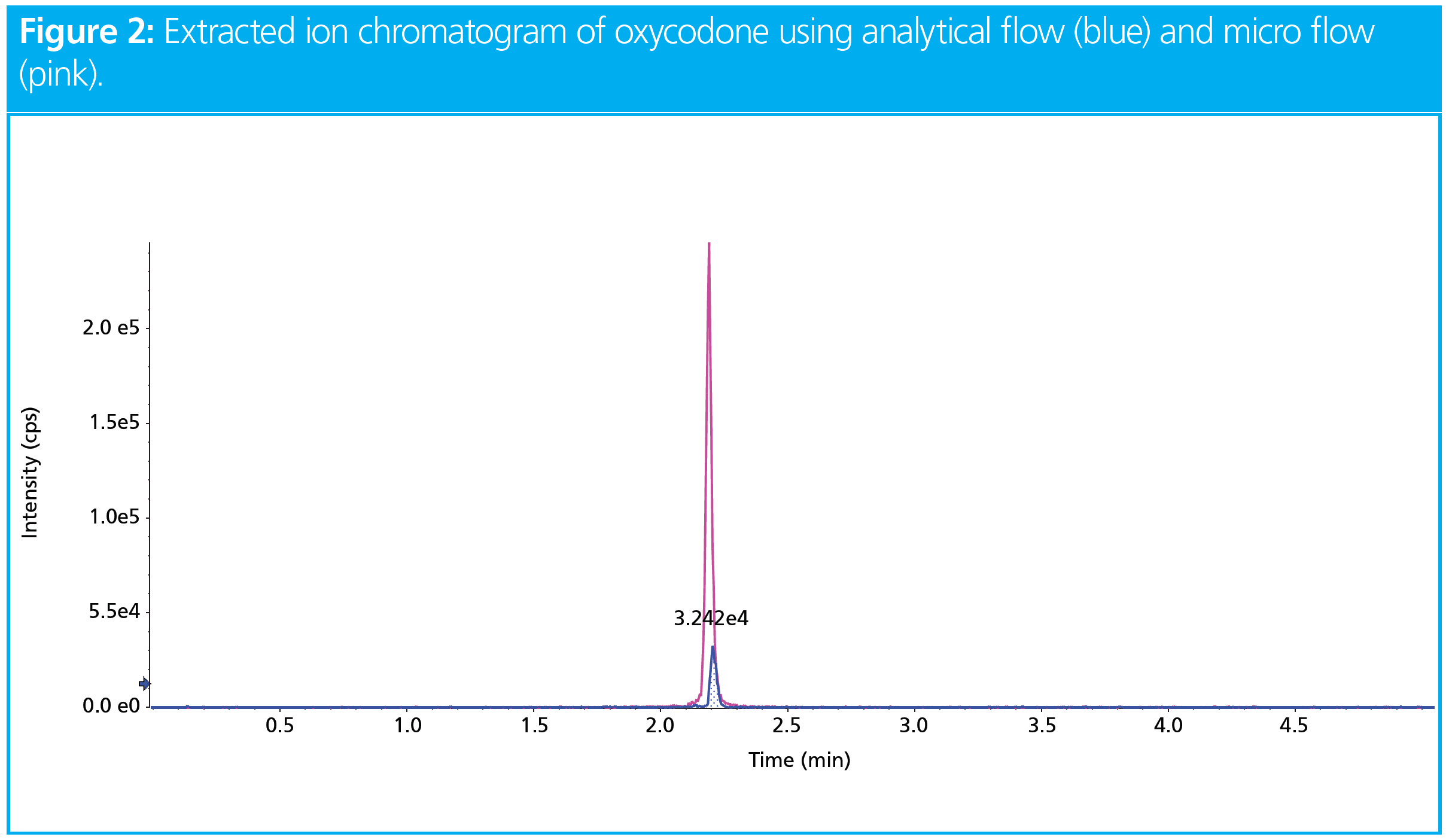
Theoretically, scaling-down the column internal diameter (i.d.) from 2.1 mm to 0.300 mm results in a sample concentration of 49-fold, which should translate into a 49‑fold increase in sensitivity (8,9). Realistically, this is not always the case; sensitivity is measured by ion intensity, which depends on other factors besides sample concentration, such as the ionization nature of the analyte, and the LC–MS equipment (emitter type) and source parameters. Nonetheless, as shown in Figure 2, scaling‑down the column inner diameter from 2.1 to 0.300 mm can result in an increase in sensitivity of almost 10‑fold for 50 ng of oxycodone.
Furthermore, calculated by equation 1:
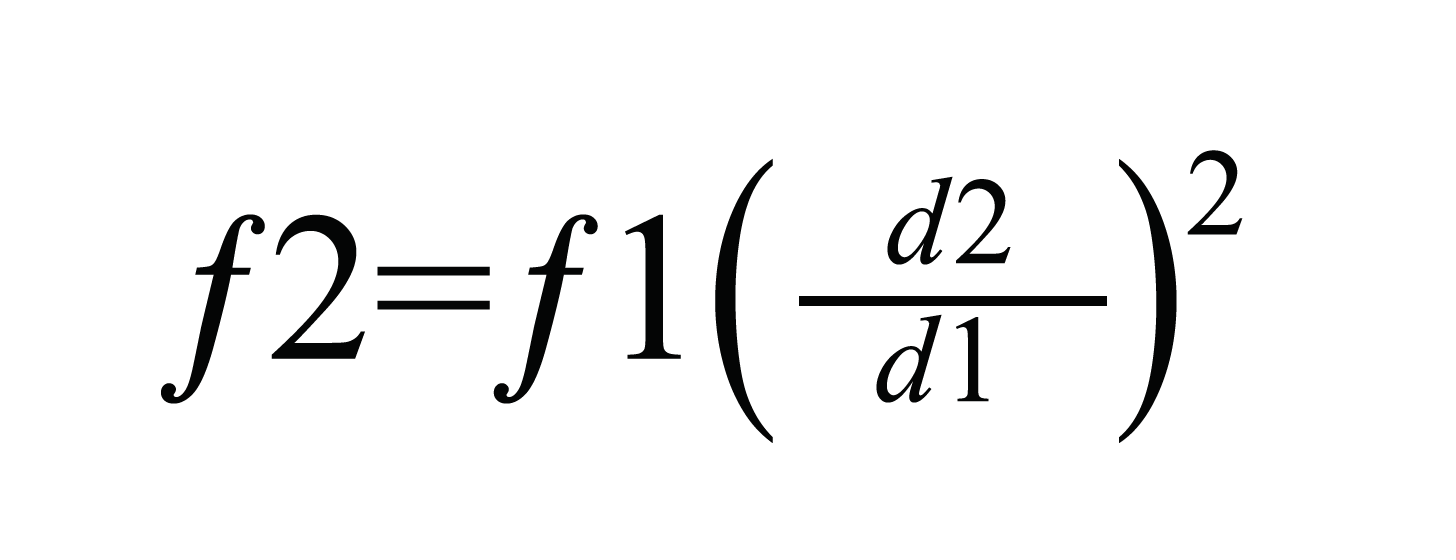
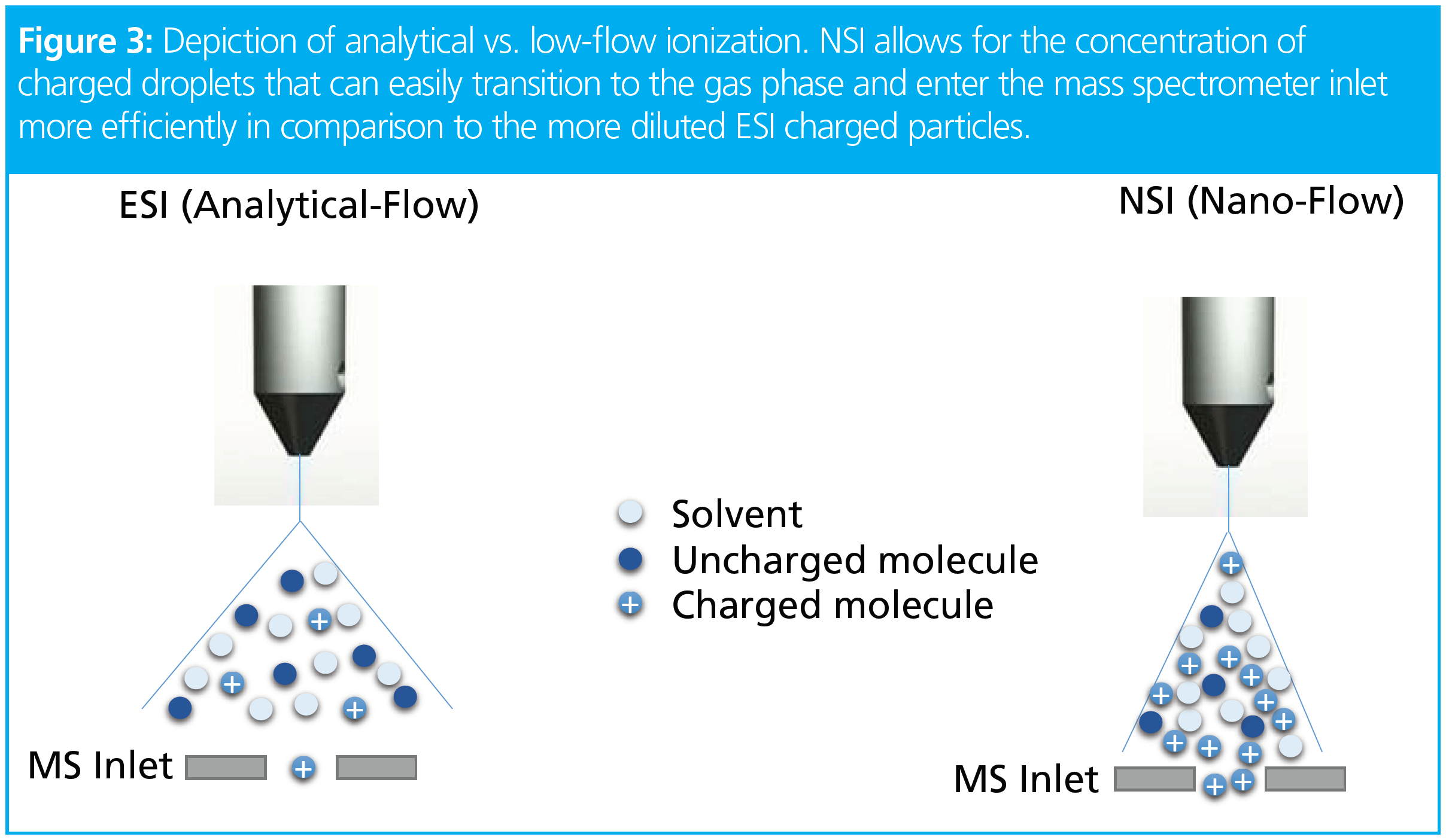
where f = flow rate and d = column inner diameter and f2>f1 and d2>d1, a decrease in column inner diameter also requires a decrease in flow rate to maintain the same (or similar) linear velocity; with a reduction in flow rate comes an increase in ionization efficiency. In mass spectrometry, electrospray ionization (ESI) is used when analytical-LC flow rates are used (Table 1). Analytical flow produces a large volume of liquid at the MS emitter, which makes ionization efficiency troublesome. Consequently, ESI uses high voltages, high temperatures, and high amounts of nebulization gases to produce gas phase ions from these large charged liquid droplets.
Flow reduction decreases droplet size at the MS emitter and increases the concentration of charged analytes (Figure 3). Typically, a voltage of 1.5–3 kV is applied, allowing for those analytes to transition from liquid to gas phase without the need of nebulizing gases, high temperatures, and high voltages that tend to hamper ionization of analytes present in low amounts. In comparison to ESI used in analytical flow, nano-ESI (NSI) produces a stable spray that can provide better sensitivity of analytes containing undesirable but perhaps necessary amounts of salts, such as in the case of discovery lipidomics (6), but more importantly it provides better sensitivity overall.
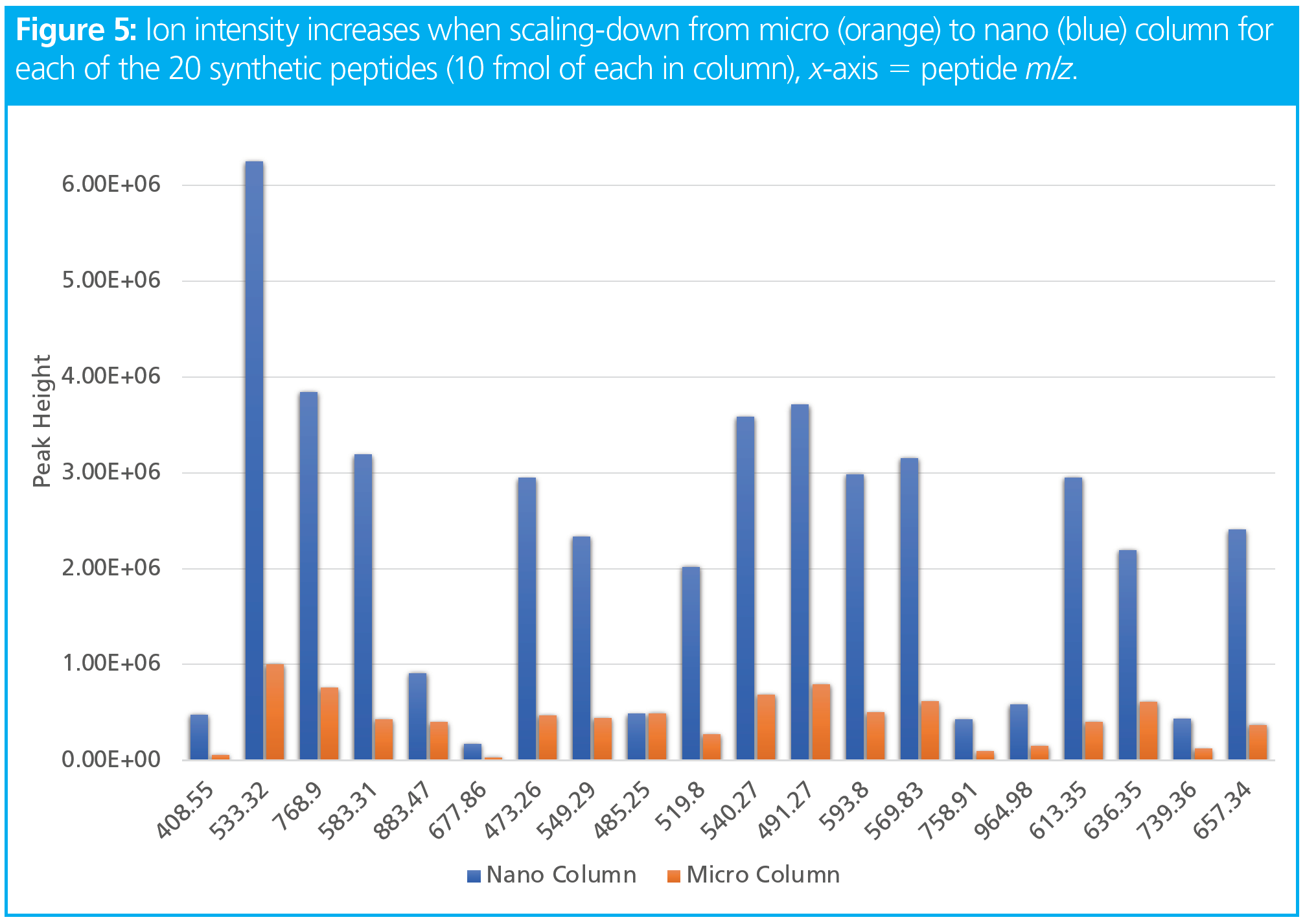
Column miniaturization reduces chromatographic dilution and greatly improves ionization efficiency. This combination makes nano-flow a highly desirable technique in the world of omics when only low amounts of sample are available and better ionization is needed because of the addition of nonvolatile solvents or salts. Besides improved sensitivity, higher signal-to-noise (S/N), and lower limits of quantitation (LLOQ), it also reduces solvent consumption, which reduces costs and benefits the environment. At the same time, with a reduction in flow rate there is sometimes the need to increase gradient length. An increase in gradient length reduces sample throughput. Nonetheless, when sample throughput, sensitivity, and robustness are desired, micro-flow is a great intermediate option (Figures 4 and 5). Figure 5 illustrates the increase in sensitivity achieved by column miniaturization when scaling-down the column inner diameter from 0.300 mm (micro-flow) to 0.075 mm (nano-flow).
Despite the many benefits of low‑flow chromatography, there are also a few considerations that must be taken into account. When moving towards nano- and micro-flow, one must consider the dwell volume. This is the volume it takes from when the gradient is formed to when it reaches the head of the column and is responsible for gradient delays. In the case of analytical flow, a dwell volume of 5 µL does not cause a noticeable gradient delay, but the same dwell volume when operating at 250 nL/min would cause a gradient delay of about 20 min. To minimize this volume, low-flow instrumentation must be plumbed with 10–75 µm i.d. capillary tubing.
Extracolumn volume refers to the volume found between the injector and the detector and is responsible for mixing effects that lower observed efficiency as it adds extracolumn broadening. It can be found in any of the components of the LC system (needle seat, frits, tubing, connections) and can contribute to peak broadening. To minimize extracolumn volume effects, low‑flow chromatography uses miniaturized LC systems in which each of its components transports only a small volume in comparison to conventional LC systems. Furthermore, to minimize or avoid extracolumn volume effects, every single connection must be routinely inspected for leaks, which can be very time-consuming. A leak in the low‑flow world is almost always undetectable by the naked eye and it requires a highly experienced operator to detect such leaks.
In addition, column clogging and overloading also need to be considered as microscopic solids or, in the case of proteomics, undigested proteins can clog the small inner diameter column, rendering it unusable. Thus, the user must be careful to properly clean the sample prior to injection and use a trap (precolumn) column to eliminate undesired contaminants from reaching the miniaturized column. When using a trap column, the trap is in line with the column, the sample is loaded into it, and undesirable particles go to waste. Once the sample is concentrated into the trap and free of contaminants, it is directed towards the column for analysis. Using a trap column can help to avoid nano- and micro-column contamination and to extend the column’s lifetime.
Column miniaturization can bring many challenges, but there is no denying that the benefits outweigh these. The increase in ion intensity brought by low-flow chromatography has made it possible to identify biomarkers for clinical research (10) and to identify thousands of proteins using less than 2 ng of protein, and hundreds in single cells (11) as well as metabolites (5). The biomedical advancements and gains in fundamental scientific knowledge brought by micro- and nano-flow separations make this a powerful analytical technique.
References
- L. Yi, P.D. Piehowski, T. Shi, R.D. Smith, and W.J. Qian, J Chromatogr A.1523, 40–48 (2017).
- E. Shishkova, A.S. Hebert, and J.J. Coon, Cell Syst. 3(4), 321–324 (2016).
- C. Aydoğan, F. Rigano, L.K. Krčmová, D.S. Chung, M. Macka, and L. Mondello, Anal Chem. 92(17), 11485–11497 (2020).
- C. Aydoğan, Trends in Analytical Chemistry 121, 1–17 (2019).
- A.J. Chetwynd and A. Davd, Talanta 182, 380–390 (2019).
- N. Danne-Rasche, C. Coman, and R. Ahrends, Anal Chem. 90(13), 8093–8101 (2018).
- A.E. El-Faramawy, S.K.W. Michael, and B.A. Thomson, American Society for Mass Spectrometry 16, 1702–1707 (2005).
- H.D. Meiring, E. van der Heeft, G.J. ten Hove, and A.P.J.M. de Jong, J. Sep. Sci. 25, 557 (2002).
- J.P.C. Vissers. J. Chrom. A 856, 117 (1999).
- S.R. Wilson, T. Vehus, H.S. Berg, and E. Lundanes, Bioanalysis 7, (2015) DOI: https://doi.org/10.4155/bio.15.92
- Y. Zhu, G. Clair, W.B. Chrisler, Y. Shen, R. Zhao, A.K. Shukla, R.J. Moore, R.S. Misra, G.S. Pryhuber, R.D. Smith, C. Ansong, and R.T. Kelly, Angew Chem Int Ed Engl. 57(38), 12370–12374 (2018).
Roxana Eggleston-Rangel is an application scientist at Phenomenex. She received her bachelors and masters degree in chemistry and biochemistry from California State University (Los Angeles, USA). Roxana is an LC–MS/MS enthusiast currently working on developing LC–MS/MS methods, with a focus on nano- and micro-flow separations.
E-mail: roxanae@phenomenex.com
Website: www.phenomenex.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.













