When van Deemter Goes Upside Down in Chiral Chromatography
The occurrence of convex-upward van Deemter curves is rare but not unusual in chiral liquid chromatography (LC). In this article, this behaviour, experimentally observed for the more retained enantiomer of a chiral sulfoxide on a polysaccharide-based chiral stationary phase (CSP), has been investigated from an experimental viewpoint, finding that this species is characterized by a very strong, localized adsorption on the CSP. The observation of the behaviour of other chiral sulfoxides with different chemical structures has suggested correlations between molecular properties and specific interactions on the CSP.
If one goes back in time and starts thinking about one of the first notions learned about chromatography, the van Deemter equation certainly comes to mind. This concept is one of the main pillars of chromatography, correlating the efficiency of a column with the mobile phase flow rate. In its adimensional version, the van Deemter equation is expressed in terms of reduced plate height (h) in equation 1:

where h is a function of the reduced interstitial velocity (ν) as in equation 2:
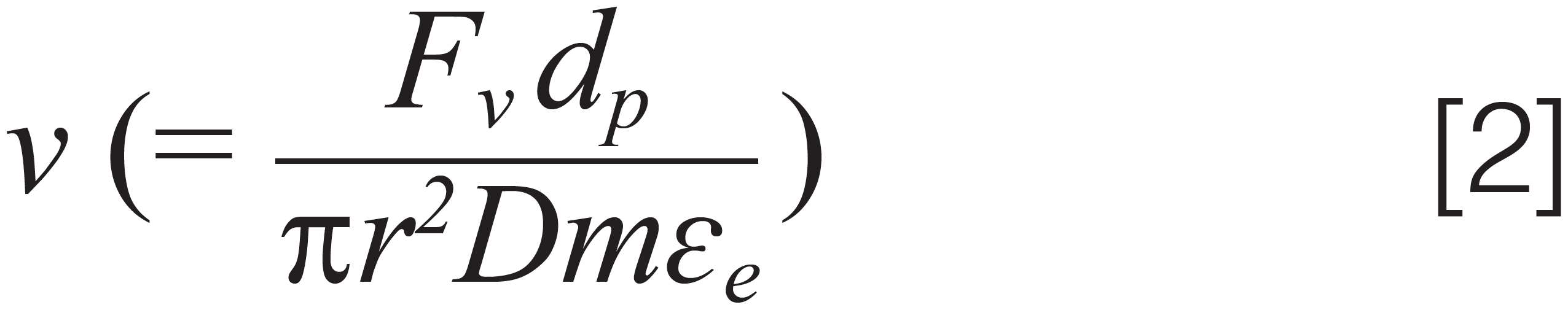
where Fv is the flow rate, dp the particle diameter, r the column radius, εe the external porosity, and Dm the bulk molecular diffusion coefficient. For liquid chromatography (LC) separations, books commonly report the van Deemter equation as the sum of three independent terms, representing the main sources of band broadening in a packed bed column:
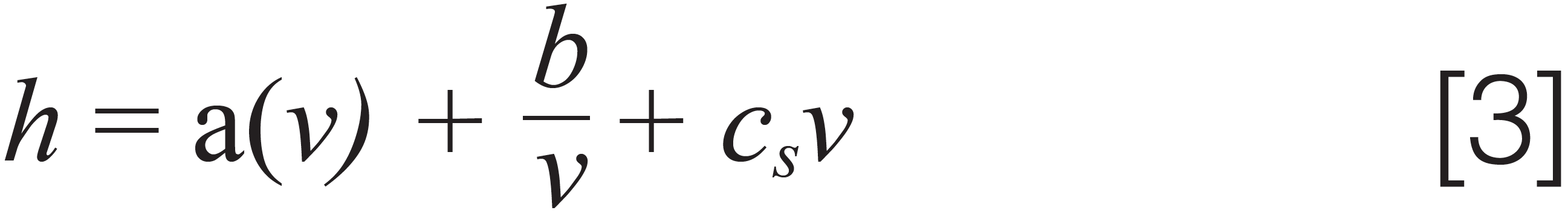
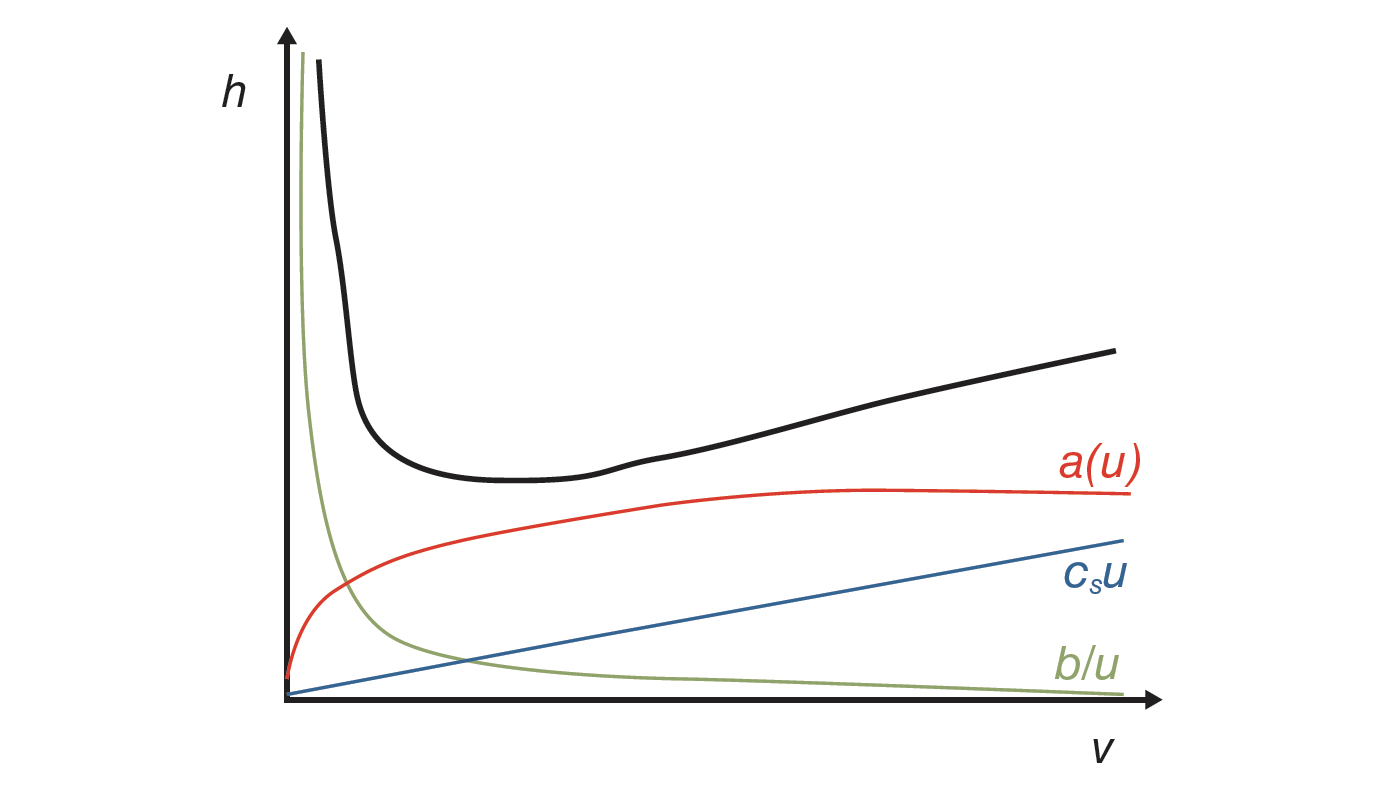
where a(ν) is the eddy dispersion term, which is related to the presence of multiple paths in the porous adsorbent, b the longitudinal diffusion, and cs the term accounting for the finite rate of mass transfer between the liquid mobile phase and the solid adsorbent (1,2). One of the most common questions for students during analytical chemistry examinations is to draw the van Deemter curve and explain its trend. The expected answer is schematically represented in Figure 1.
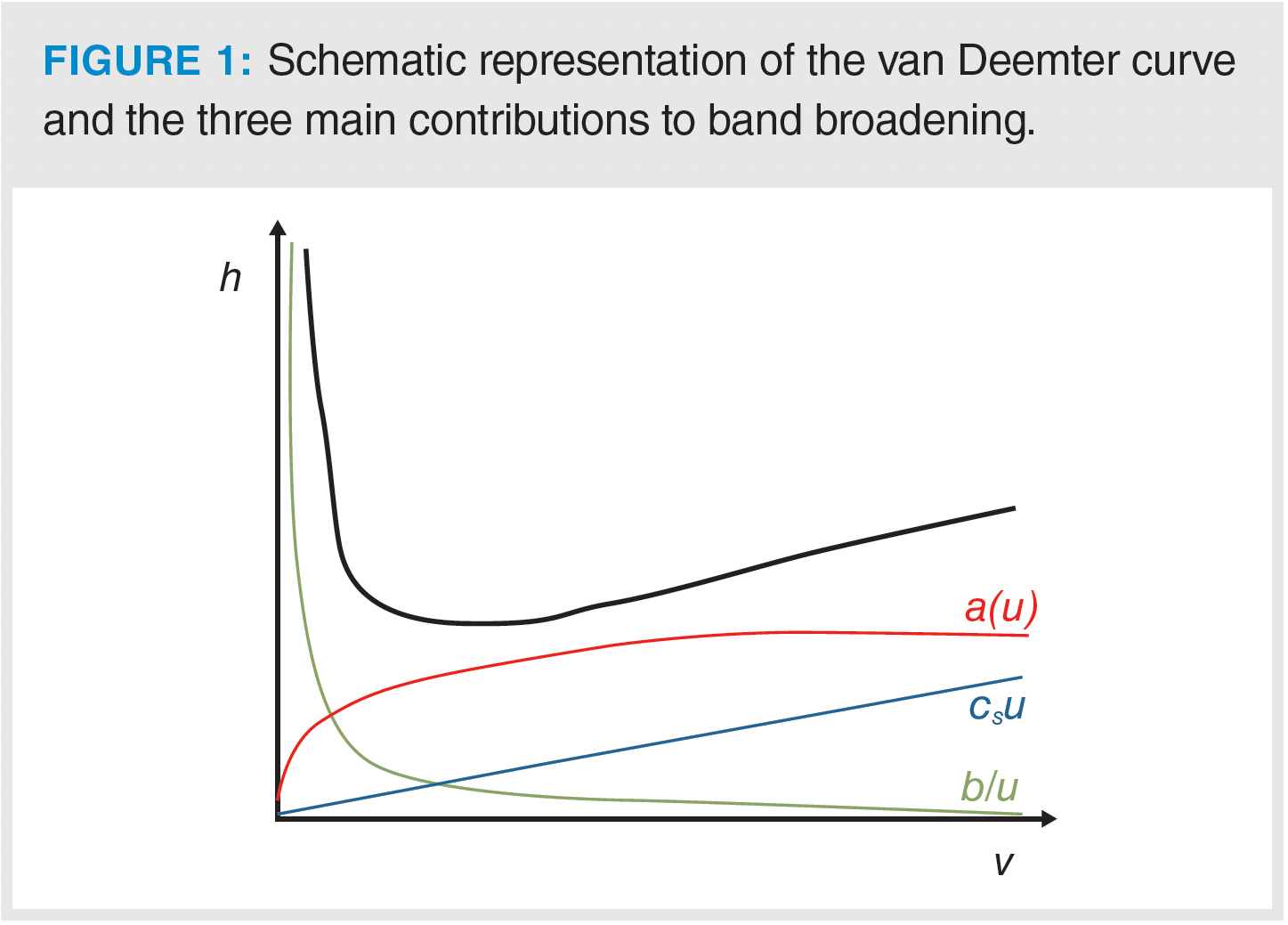
The sum of the three terms of equation 3 gives place to the common convex‑downward curve, with a minimum corresponding to the optimal conditions—that is, where the best performance can be achieved in terms of efficiency. Indeed, the impact of longitudinal diffusion prevails at low flow rates, while at high flow rates (c-branch), the main source of band broadening is given by the solid–liquid mass transfer term. These three terms can be independently estimated by conducting stop-flow measurements, that is, peak parking (3,4) for the estimation of diffusion coefficients, and by interpreting results in light of a proper model of diffusion in porous media (5–7).
This behaviour is generally applicable to the separation of small molecules in reversed-phase LC, but in other circumstances other sources of band broadening could arise from a more complex retention mechanism. This is the case, for instance, for the considerable loss of efficiency at high flow rates due to the presence of frictional heating effects, especially when using columns packed with small particles (for example, sub‑2‑μm), or because of the impact of slow adsorption-desorption kinetics. This phenomenon is usually negligible, but, in some cases, it is one of the main sources of band broadening, for instance, for separations of large biomolecules and in chiral chromatography, especially for the second eluted enantiomer (8). This extra source of band broadening usually leads to an extra term in equation 3. This term is directly proportional to the mobile phase velocity and is therefore written as cadsν. Its independent estimation is very difficult to establish, and even the most advanced approaches are not suitable to directly determine it (8,9).
Occurrence of Convex-Upward van Deemter Curves in Chiral Chromatography
Enantiorecognition is a very complex mechanism and mass transfer phenomena in chiral chromatography are not yet completely understood. In the last 10 years, several authors have studied kinetic performance and diffusion processes in chiral chromatography (9–18) by observing an unusual phenomenon where the van Deemter curve of the first enantiomer has the typical shape, while that of the second one is convex-upward instead of convex-downward. This behaviour was first reported by Armstrong and co-workers (11) for the separation of L- and D-m-tyrosine and L- and D-homophenylalanine on macrocyclic antibiotic chiral stationary phases (CSPs) (ristocetin and teicoplanin, respectively) made on 2.7-μm superfically porous particles (SPPs) using water–methanol mobile phases. The authors explained this behaviour through the impact of the frictional heating effect, which negatively affects efficiency by developing axial and radial temperature gradients.
A similar behaviour was also observed by Asnin and co‑workers (10) for the separation of pyrroloquinolone enantiomers on a ristocetin CSP using a mixture of water–acetonitrile as mobile phase. In this case, the authors explained these findings by considering the large retention of the second enantiomer on the CSP, as well as the impact of an imperfect packing, which negatively affected eddy dispersion, especially for the second eluted enantiomer. In addition, they also mentioned a possible influence of slow adsorption-desorption kinetics.
In the present study, we have also observed convex‑upward van Deemter curves, as shown in Figure 2, by using two non-commercial chiral sulfoxides on a polysaccharide (cellulose-based) CSP, prepared by covalently immobilizing tris(4-chloro-3-methylphenylcarbamate) on fully porous particles (FPPs) of 3.0 μm in diameter and 1000 Å pore size (17).

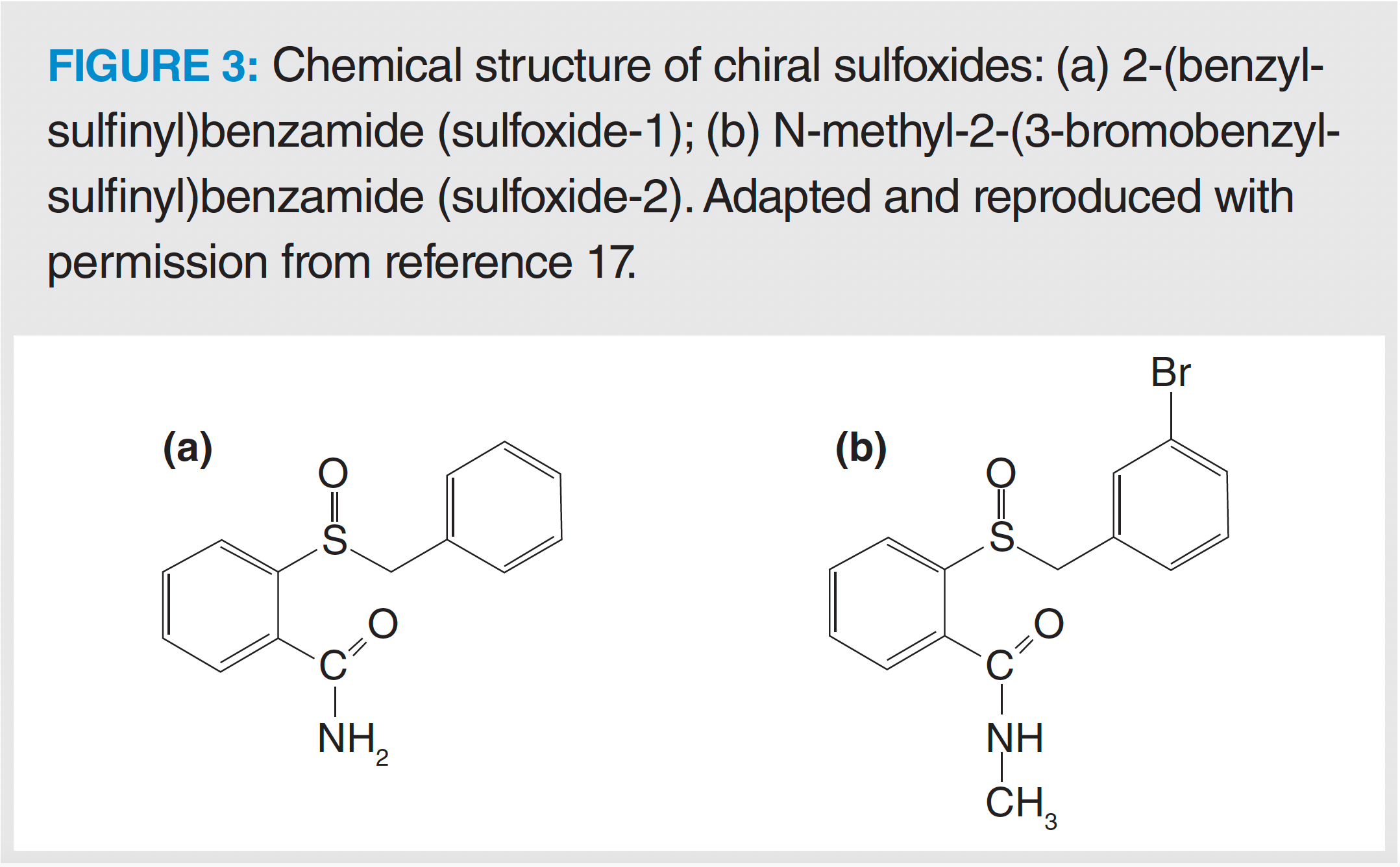
The chemical structures of the two analytes are shown in Figure 3, where the two molecules differ only in two substituents, a methyl and a halogen. This led to a completely different kinetic and retention behaviour for the most retained enantiomers, but to almost the same trend for the first eluted enantiomers.
Indeed, as clearly shown in Figure 2, the second eluted enantiomer of sulfoxide-2 shows an unusual trend of the van Deemter curve, with a 12 times larger retention factor (k = 6.0) when compared to the second eluted enantiomer of sulfoxide-1 (k = 0.5).
This change in efficiency, retention, and selectivity has been investigated by coupling stop-flow measurements to estimate diffusion coefficients of enantiomers in the porous media with the calculation of adsorption isotherms. This approach has revealed that the second eluted enantiomer of sulfoxide-1 has one order of magnitude smaller effective diffusion coefficient with respect to the first one, indicating a very limited diffusion into the porous CSP, which also translates into a 50% smaller b-term (equation 3). By interpreting these findings with a proper model of diffusion in porous media, it was possible to both reveal that surface diffusion for this analyte was negligible and to calculate the solid–liquid mass transfer coefficients, cs-term (equation 3), for all the species. It was found that the impact of solid–liquid mass transfer resistance was higher for the second eluted enantiomer of sulfoxide-1 with respect to all the other enantiomers, but this was expected since the impact of surface diffusion was negligible for this species. However, this difference in cs‑term could not be the only reason for the observed behaviour, especially considering that the second eluted enantiomer was strongly retained on the CSP, as observed in Figure 2.
The investigation of the adsorption behaviour of the enantiomers on the CSP allowed important information on their retention mechanism to be obtained. Adsorption isotherms were described through the Bi-Langmuir model, which takes into account the presence of both chiral (selective) and achiral (non-selective) sites on the CSP. It was found that the binding constant (which gives an estimation of the “strength” of adsorption) of the second eluted enantiomer of sulfoxide-1 on selective sites was 80 times larger than that of its less retained enantiomer, and 15 times larger than that of the more retained sulfoxide-2 enantiomer. These findings suggest, on the one hand, that adsorption-desorption kinetics could have a great influence on the unusually large loss of efficiency observed for the second eluted enantiomer of sulfoxide-1. Indeed, it is commonly acknowledged that large binding constants are directly linked to slower adsorption-desorption processes (8). On the other hand, these results support the hypothesis that adsorption is localized.
From a molecular point of view, the different behaviour between the two sulfoxides can be explained by considering their individual substituents. Indeed, the presence of an electron-donating substituent (methyl group) on the amide function of sulfoxide-2 hinders the possibility of establishing hydrogen bonds (19,20), while the (electron-attracting) bromine on the benzyl group could limit the π-π donor ability of the molecule. This translates into a weaker interaction between sulfoxide-2 and the active adsorption sites of the chiral selector. Conversely, the most retained enantiomer of sulfoxide-1 is able to fully interact with the CSP by means of specific hydrogen bonding and other noncovalent interactions. This perfect matching between the chiral molecule and the active portion of the CSP, where enantiorecognition takes place, leads to a strong binding (and thus large retention) and a very low adsorption-desorption process, with a detrimental effect on efficiency of the most retained enantiomer.
Conclusions
Convex-upward van Deemter curves represent an interesting yet challenging phenomenon occurring in the separation of large biomolecules or in chiral chromatography, especially for the most retained enantiomer. This rare but not unusual phenomenon is most likely due to a combination of both kinetic and thermodynamic factors, depending on the nature of the analyte and on its possibility of interacting with the selective portion of the CSP. According to our investigations, very large binding constants and localized adsorption are the main contributions that negatively affect efficiency. However, further investigation is required to understand the possible impact of eddy diffusion, which is very complicated to establish.
These findings are fundamental to deepening our understanding of the complex enantiorecognition mechanism. Chiral LC is, indeed, one of the fields where, especially in recent years, there has been an increasing push towards ultrafast separations, for which high efficiency and rapid mass transfer are necessary. In addition, this phenomenon clearly demonstrates that the study of kinetic performance alone cannot provide a complete understanding of the separation process, which could be strongly affected by thermodynamic phenomena and by specific interactions of analytes with CSPs. Indeed, it cannot be excluded that when chiral selectors are immobilized on silica, they could arrange in such a way that chiral cavities are present and only molecules with specific steric arrangements (also within the same species) could fit them.
References
(1) Skoog, D.A.; West, D.M.; Holler, F.J.; Crouch, S.R. Fundamentals of Analytical Chemistry, 6th ed.; Saunders College Pub., 2014.
(2) Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis, 6th ed.; Thomson Brooks/Cole, 2017.
(3) Miyabe, K.; Matsumoto, Y.; Guiochon, G. Peak Parking-Moment Analysis. A Strategy for the Study of the Mass-Transfer Kinetics in the Stationary Phase. Anal. Chem. 2007, 79, 1970–1982. DOI: 10.1021/AC061321H
(4) Song, H.; Sadriaj, D.; Desmet, G.; Cabooter, D. Methodologies to determine b-term coefficients revisited. J. Chromatogr. A 2018, 1532, 124–135. DOI: 10.1016/j.chroma.2017.11.070
(5) Desmet, G.; Deridder, S. Effective medium theory expressions for the effective diffusion in chromatographic beds filled with porous, non-porous and porous-shell particles and cylinders. Part I: Theory. J. Chromatogr. A 2011, 1218, 32–45. DOI: 10.1016/j.chroma.2010.10.087
(6) Liekens, A.; Denayer, J.; Desmet, G. Experimental investigation of the difference in B-term dominated band broadening between fully porous and porous-shell particles for liquid chromatography using the Effective Medium Theory. J. Chromatogr. A 2011, 1218, 4406–4416. DOI: 10.1016/j.chroma.2011.05.018
(7) Catani, M.; Ismail O.H.; Cavazzini, A; et al. Rationale behind the optimum efficiency of columns packed with new 1.9 μm fully porous particles of narrow particle size distribution. J. Chromatogr. A 2016, 1454, 78–85. DOI: 10.1016/j.chroma.2016.05.037
(8) Gritti, F.; Guiochon, G. Mass transfer kinetics, band broadening and column efficiency. J. Chromatogr. A 2012, 1221, 2–40. DOI: 10.1016/j.chroma.2011.04.058
(9) Gritti, F.; Guiochon, G. Mass transfer mechanism in chiral reversed phase liquid chromatography. J. Chromatogr. A 2014, 1332, 35–45. DOI: 10.1016/J.CHROMA.2014.01.040
(10) Asnin, L.D.; Boteva, A.A; Krasnykh, O.P.; Stepanova, M.V.; Ali, I. Unusual van Deemter plots of optical isomers on a chiral brush-type liquid chromatography column. J. Chromatogr. A 2019, 1592, 112–121. DOI: 10.1016/J.CHROMA.2019.01.048
(11) Patel, D.C.; Breitbach, Z.S.; Wahab, M.F.; Barhate, C.L.; Armstrong, D.W. Gone in Seconds: Praxis, Performance, and Peculiarities of Ultrafast Chiral Liquid Chromatography with Superficially Porous Particles. Anal. Chem. 2015, 87, 9137–9148. DOI: 10.1021/ACS.ANALCHEM.5B00715
(12) Patel, D.C.; Breitbach, Z.S.; Yu, J.J.; Nguyen, K.A.; Armstrong, D.W. Quinine bonded to superficially porous particles for high-efficiency and ultrafast liquid and supercritical fluid chromatography. Anal. Chim. Acta. 2017, 963, 164–174. DOI: 10.1016/J.ACA.2017.02.005
(13) Geibel, C.; Dittrich, K.; Woiwode, U.; et al. Evaluation of superficially porous particle based zwitterionic chiral ion exchangers against fully porous particle benchmarks for enantioselective ultra-high performance liquid chromatography. J. Chromatogr. A 2019, 1603, 130–140. DOI: 10.1016/J.CHROMA.2019.06.026
(14) Catani, M.; Ismail, O.H.; Gasparrini, F.; et al. Recent advancements and future directions of superficially porous chiral stationary phases for ultrafast high-performance enantioseparations. Analyst 2017, 142, 555–566. DOI: 10.1039/C6AN02530G
(15) Berger, T.A. Kinetic performance of a 50 mm long 1.8 μm chiral column in supercritical fluid chromatography. J. Chromatogr. A 2016, 1459, 136–144. DOI: 10.1016/J.CHROMA.2016.07.012
(16) Schmitt, K.; Woiwode, U.; Kohout, M.; et al. Comparison of small size fully porous particles and superficially porous particles of chiral anion-exchange type stationary phases in ultra-high performance liquid chromatography: effect of particle and pore size on chromatographic efficiency and kinetic performance. J. Chromatogr. A 2018, 1569, 149–159. DOI: 10.1016/J.CHROMA.2018.07.056
(17) Felletti, S.; De Luca, C.; Lievore, G.; et al. Shedding light on mechanisms leading to convex-upward van Deemter curves on a cellulose tris(4-chloro-3-methylphenylcarbamate)-based chiral stationary phase. J. Chromatogr. A 2020, 1630, 461532. DOI: 10.1016/j.chroma.2020.461532
(18) Kharaishvili, Q.; Jibuti, G.; Farkas, T.; Chankvetadze, B. Further proof to the utility of polysaccharide-based chiral selectors in combination with superficially porous silica particles as effective chiral stationary phases for separation of enantiomers in high-performance liquid chromatography. J. Chromatogr. A 2016, 1467, 163–168. DOI: 10.1016/J.CHROMA.2016.08.046
(19) Khundadze, N.; Pantsulaia, S.; Fanali, C.; Farkas, T.; Chankvetadze, B. On our way to sub-second separations of enantiomers in high-performance liquid chromatography. J. Chromatogr. A 2018, 1572, 37–43. DOI: 10.1016/j.chroma.2018.08.027
(20) Bezhitashvili, L.; Bardavelidze, A.; Mskhiladze, A.; et al. Application of cellulose 3,5-dichlorophenylcarbamate covalently immobilized on superficially porous silica for the separation of enantiomers in high-performance liquid chromatography. J. Chromatogr. A 2018, 1571, 132–139. DOI: 10.1016/j.chroma.2018.08.011
Martina Catani is an assistant professor in analytical chemistry at the University of Ferrara (Dept. of Chemical, Pharmaceutical, and Agricultural Sciences). She received a Ph.D. in chemical sciences in 2018 from the same university.
Alberto Cavazzini has been a full professor of analytical chemistry in the Department of Chemical, Pharmaceutical, and Agricultural Sciences at the University of Ferrara since 2014. He was research fellow at the University of Tennessee, Knoxville, USA, and Oak Ridge National Laboratories, Oak Ridge in Tennessee (2000–2002), in the group of Prof. Georges Guiochon.
Chiara De Luca received her Ph.D. degree in chemical sciences at the University of Ferrara in 2021. She is currently a postdoc at the Department of Chemical, Pharmaceutical, and Agricultural Sciences at the University of Ferrara.
Simona Felletti received her Ph.D. degree in 2020. She is currently a postdoc at the Department of Chemical, Pharmaceutical, and Agricultural Sciences at the University of Ferrara.

Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)