What LC Troubleshooting Has Taught Me
Special Issues
John shares lessons learned from his more than three decades of teaching liquid chromatography practitioners and helping them solve their problems.
John shares lessons learned from his more than three decades of teaching liquid chromatography practitioners and helping them solve their problems.
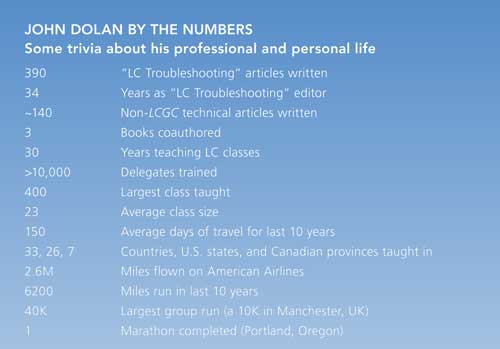
I was the editor of “LC Troubleshooting” for 34 years, from October 1983 to October 2017. During most of that time, I’ve been a principal at LC Resources, a company dedicated in part to training workers in all aspects of liquid chromatography (LC) including basics, method development, and troubleshooting. Over this time I’ve taught more than 10,000 students in 33 countries. As a result, I’ve had the rare privilege of seeing LC in action in some of the best and some of the worst laboratories in the world. I worked for two instrument companies before joining LC Resources. In my job at LC Resources, I wrote the initial versions of the popular DryLab method development software (Molnar Institute), managed our analytical laboratory, and taught LC training classes. As “LC Troubleshooting” editor, I’ve had several thousand interactions with readers as I’ve tried to help you solve your problems. These experiences gave me a rich diversity of LC troubleshooting problems and solutions that I’ve enjoyed sharing with you, the readers of LCGC, over the years.
My father was a mathematician, and my wife claims that it infected me with a math gene. I subconsciously find myself counting my pace as I run (1426 steps/mile at last check), stairs I climb, the number of train cars passing by (average of about 100/train in Oregon), and nearly anything else. I tend to naturally count, categorize, and try to simplify things into digestible and easy to remember chunks. This is reflected in my teaching style and the content of my “LC Troubleshooting” articles. In selecting columns for this special issue, I’ve chosen ones that represent some of those numeric condensations. These are rules of thumb, tricks of the trade, or best practices that have helped me to diagnose, correct, and prevent LC problems. I hope they will be useful reminders for you that the majority of LC-related problems fall into a few categories and that they can be identified, corrected, or avoided using one or more of the techniques discussed here. All of my columns from 2000 onward are available at LCGC’s website (www.chromatographyonline.com); if you are interested in earlier ones, they can be found in The “LC Troubleshooting Bible” on the LC Resources website (www.lcresources.com/tsbible).
In the present discussion, I’m backing off and taking a rather broad view of the overall LC process. As is apparent from the “LC Troubleshooting” articles that have been reprinted in this special issue, there is a fairly short list of best practices that will help to ensure success in the LC laboratory. In the present discussion, I’d like to further distill these distillations of what I’ve learned in the last 45 years since I made my first LC injection in 1972. I’ll concentrate on three main areas: the methodology, the instrumentation, and the operator. Hopefully, it won’t take you 45 years to reach similar conclusions.
Methodology: Be Consistent
Consistent action creates consistent results,” is a quote, commonly attributed to Christine Kane, that we may see on an inspirational poster in the training room. As analytical chemists, consistency is at the core of our being. We run calibration standards at the beginning of a sample batch and often at the end, we intersperse standards and check samples throughout the run. Our very reputations and businesses live or die on the consistency of the analytical results we produce. Consistent results tend to be trustworthy. So in all we do, we strive for consistency.
Properly developing, validating, documenting, and using an LC method are parts of analytical consistency. In the pharmaceutical field, as well as many other industries under regulatory oversight, we have guidelines to help us with each of these steps and the promise of occasional regulatory inspections to help ensure we follow the guidelines. To be honest, until I began to work in a laboratory operating under these requirements, I wasn’t consistent. My laboratory notebook was embarrassing, my lab technique was sloppy, and the results were often inconsistent. After I made the transition to learning and using standard operating procedures (SOPs), I found there was trickle-down in better performance in all aspects of my work-and many fewer problems. The changeover process was painful, but now I wonder how I ever functioned without it.
In routine use, the method document should be our guide. After you’ve run an LC method a few times, you begin to know it intimately and this is reflected in the efficiency of your setup and execution of the analysis. Pretty soon you know most of the details by heart-the mobile-phase composition, the flow rate, the column, and so forth. In my experience, this is where most method problems begin-relying on memory. I think part of this sloppy work technique is our own fault based on the way the method is written. The method may be complete, with all the details, but it also needs to be written in a way that makes it convenient to use. I find that a summary table of conditions or other simplification of the method content makes it much easier to use. Another trick is to make a method setup form to use that summarizes the important details to follow.
If you make a good habit of following all the method details, you are much less likely to encounter method-related problems. And when you do have problems, you can automatically eliminate many causes if you can confirm that your method setup checklist was followed and can verify weights and volumes by looking at your laboratory notebook records.
Instrumentation: Be Good to It
Today’s LC instruments are greatly advanced from those available in the early 1970s when I began my LC career. Most of the instrument problems we encountered in the early days are only memories with today’s equipment. Not only are the instruments more reliable, but they also have many built-in monitoring systems to help track performance. However, just as was mentioned in the previous discussion about methods, success can lead to sloppiness. If we get used to having reliable instrumentation, we may get lazy about routine preventive maintenance that will ensure long-term reliability.
I’m reminded of an LC–tandem mass spectrometry (LC–MS/MS) system we inherited in our laboratory from a lab that had been closed. It was the same make and model as three other LC–MS/MS units we had, so we were excited about getting another one because the others had been so reliable. We performed a routine preventive maintenance program on the new instrument before putting it into use. Among other things, this program comprised cleaning the interface and the first quadrupole plus changing the oil in the roughing pumps. During the cleaning, we observed loose screws and other indications that prior maintenance was not up to our standards. When changing the pump oil, we noticed that it looked much darker than we were used to, suggesting that regular oil changes were not part of the past routine, either. When we ran instrument checks after the preventive maintenance session, we discovered that the entire system was still dirty, including the collision cell and the third quadrupole. Our senior MS scientist had been doing LC–MS for more than 20 years and had never seen a dirty collision cell, let alone the third quadrupole. It appeared that the first quadrupole had been allowed to get so dirty that it no longer stopped contaminants from making it further downstream. This is analogous to using a guard column on the LC, but never changing it-eventually it will get overloaded and no longer do its work.
One problem with instrumental sloppiness is that the degradation of instrument performance may come very slowly and in small increments that may not be noticeable from day to day. Then one day you realize you have a problem, but it may have begun weeks or months earlier because of poor laboratory practice. The past columns selected for this special issue should give you some ideas about what things you should do on a routine basis to minimize instrumental downtime. Here are a few items that I’ve found most helpful to me:
· Keep the dirt out. The nature of chemical analysis is that we have to put some things into the column that aren’t good for it, but we can take care to minimize this. Used appropriately, sample cleanup, solvent filtering, in-line filters, and guard columns will help to minimize the contamination of the instrument and column.
· Flush the system with strong solvent after each sample batch. This step will remove strongly retained materials from the column and will leave the instrument and column in a buffer-free mobile phase, which has a low likelihood of being corrosive or supporting microbial growth.
· Dedicate a column to each method. Use a single column for every method instead of sharing columns between methods to help avoid problems related to cross-contamination and a vague column history.
· Regularly replace wear parts. Different instruments and methods may require more or less frequent attention, but all will benefit from an annual preventive maintenance session. Many will need interim replacement of parts such as guard columns, columns, in-line filters, reservoir inlet frits, and various seals. Know your system and what it characteristically needs, then create and follow a preventive maintenance schedule that will replace parts before they fail.
· Pay attention to the solvents. Be sure to replace the solvents, especially buffers, before they degrade. I find that replacing buffers once a week for conventional LC systems and daily for ultrahigh-pressure LC (UHPLC) systems is a simple and inexpensive practice that goes a long way toward avoiding system contamination. Organic solvents, such as methanol or acetonitrile, can be used for longer periods-I like a one-month expiration date on these. And don’t forget to replace or wash the solvent reservoirs with each solvent change. Don’t “top up” the reservoirs, especially the buffer; this practice will help to avoid cross-contamination from prior batches.
Operator: Never Stop Learning
For some of us, chromatography or analytical chemistry is the center of our lives and we thrive on it. But let’s face it, for the rest of us the laboratory is just a place to earn a paycheck. Sure, we enjoy the work, but our priorities lie outside work. Both of these approaches have their pros and cons; however, it is easy in either case to get stale in our jobs.
Some would argue that LC is a mature field, and certainly the trend line plotting new developments over time is leveling off, but new information is available regularly. Health care providers can be in a similar situation, with predictable (boring?) clientele and daily activities, but they are required to regularly attend refresher courses to “tune-up” their skills and build new ones. It is too bad that similar requirements are not placed on those of us working in the analytical lab. Instead, we need to discipline ourselves to undergo such training as a part of our own professional development. Some of you may be fortunate enough that your company pays your way to an annual conference or training program. Others must rely on free or low-priced training available live from instrument vendors or on the internet. Webinars and other resources can be a great way to follow current developments, build a skill in a new area, or refresh our memories about chromatography basics and troubleshooting. Reading LCGC and other free publications is another learning route. Building some of this learning into a regular habit, even if it has to be on your own time, will make you a more valuable employee (think raises and promotions!), allow you to more quickly solve problems, and give you more job satisfaction.
By the Numbers
As I mentioned in the introduction, I’m a numbers-oriented guy, so I picked past columns that highlighted this characteristic. Each column either has a number in the title or implicit in the content. It was fun for me to read through these columns, some nearly 20 years old. I’ve made few comments on each one here-be sure to read the full article as well.
Rules of Thumb (1988)
It is interesting that in this early column I listed five rules of thumb for reliable LC operation. I still use this list with only minor modification in the troubleshooting classes I teach. Today, I’ve incorporated the fourth “Throw It Away” rule into the third “Put It Back” rule and replaced it with a “Crystal Ball” rule that moves the material predicting failure from the last “Write It Down” rule. Still good advice today.
LC Columns-The Top-10 List (2003)
The column often is considered the most expensive consumable we buy, but if we amortize it over the number of samples run and consider the per-sample cost of analysis, the cost of the column is trivial. However, most of us would like to get the most from our column. All 10 items from this 2003 article are still good to put into practice today for a conventional system or a UHPLC system.
My Favorite Shortcuts (2004)
The number is missing from the title-it probably should say “My Nine Favorite Shortcuts” to be included in the current discussion. I have found that if I can do a bit of mental “guesstimating” instead of an actual calculation, I’m more likely to do it in practice. This discussion includes several of those mental shortcuts that I have found to be indispensable. These, too, have been popular take-aways in my classes over the years. Incorporate them in your daily habits and I think you’ll benefit, too.
A Baker’s Dozen (2005)
Although this list of 13 practices is targeted toward gradient methods, most are equally beneficial for isocratic ones. If you are reading these columns in sequence, you’ll begin to notice some repetition with some items repeated between articles. Rather than being bored with the same information again, perhaps this repetition should raise the “alert” flag that maybe something is important enough to persist from one discussion to the next. Two items in this category that popped out to me were “2. System Cleanliness” and “6. Dedicated Columns.”
Seven Things to Avoid (2015)
I always get a little laugh in my classes when I mention the “DDT Rule” . . . as in Don’t Do That! This column provides a list of seven problems that can be avoided by some simple laboratory practices, and highlights some of the reasons why many laboratories put together a “Best Practices” list to aid in obtaining consistent analytical results and minimizing downtime due to avoidable problems.
Only Three Things (2008)
As you’ll read in the introduction of this article, researchers have found that most learners take away only three things from a full-day class. So, it is an appropriate end summarizing my 34-year run of 390 “LC Troubleshooting” articles with the big three laboratory practices that will minimize your LC problems: degas, filter, and flush.

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.













