What a GC–MS Tune Report Can Tell You
LCGC Europe
An excerpt from LCGC’s professional development platform, CHROMacademy.com
An excerpt from LCGC’s professional development platform, CHROMacademy.com

Tuning in gas chromatography–mass spectrometry (GC–MS) involves adjusting several mass spectrometer parameters through the infusion of a tune compound, commonly perfluorotributylamine (PFTBA). The tune report generated is invaluable because it indicates how well the instrument is operating, and is an essential tool when troubleshooting is required. A typical autotune report is shown in Figure 1.
There are several parameters that need to be examined in this autotune report:
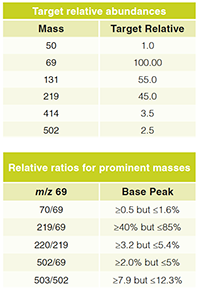
- Correct mass assignments. Check that the tune has correctly assigned the masses of peaks 69 (base peak), 219, and 502. The tolerance for unit mass resolution are: 69 (68.8–69.2), 219 (218.8–219.2), 502 (501.8–502.2).
- The mass peak widths (PW50) should be 0.55±0.1.
- Peaks should be smooth and Gaussian. Do not be concerned with peak shoulders unless they become a major component of the spectral peak. The shoulders should increase in area to reflect the isotopic abundance of C13.
- Note the electron multiplier (EM) voltage. A clean source with a relatively new EM horn should report a gain voltage of 1400 to 1600. As the lifetime of the dynode increases and the source becomes contaminated, this value may approach values of 3000 V. If the voltage consistently needs to be ramped to 2800–3000 V, it may be time for a source clean or a new dynode.
- Low background. The presence of a large number of peaks across the spectrum is known as a high background. This can arise from several sources of contamination, such as column bleed, septum bleed, oil contamination, and various other sources.
- Low water and air. Presence of large peaks at 18, 28, or 32 indicate an air or water leak.
- The tables below give the relative abundances of each of the ions, as well as isotopic masses, abundances, and ratios. The isotopic mass should always be an Mï«1 ion, for example, 70, 220, and 503 amu. As PFTBA contains only C13 and N15, the relative isotopic ratios can be easily calculated and should be approximately 69 (1.0), 219 (4.0), and 502 (10.0). Any drift in these ratios can indicate that the spectrometer mass axis has not been correctly calibrated, or that the system resolution is grossly degraded. The following conditions are typical if everything is functioning correctly. The absolute abundance of mass 69 should be ≥200,000 but ≤400,000. For a maximum sensitivity autotune, the relative ratios of each of the peaks should be within the following ranges: 219/69 (20–35%) and 502/69 (0.51%).
- Proper absolute abundance.
- The data system will allow the operator to perform an air and water check to quantify the amounts within the spectrometer at any given point in time. This can be a useful tool to check the vacuum system and spectrometer operating temperatures are equilibrated, or to check if a suspected leak has been properly fixed.
All other parameters within the autotune will differ from instrument to instrument, but general trends from subsequent autotune reports should be noted. Any consistent drift in either the positive or negative direction should be noted and then re-autotune, manual tune, or maintenance should be carried out accordingly.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Quantifying Terpenes in Hydrodistilled Cannabis sativa Essential Oil with GC-MS
April 21st 2025A recent study conducted at the University of Georgia, (Athens, Georgia) presented a validated method for quantifying 18 terpenes in Cannabis sativa essential oil, extracted via hydrodistillation. The method, utilizing gas chromatography–mass spectrometry (GC–MS) with selected ion monitoring (SIM), includes using internal standards (n-tridecane and octadecane) for accurate analysis, with key validation parameters—such as specificity, accuracy, precision, and detection limits—thoroughly assessed. LCGC International spoke to Noelle Joy of the University of Georgia, corresponding author of this paper discussing the method, about its creation and benefits it offers the analytical community.
Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.






![LCE0918_Liu Bio[2]-1.jpg LCE0918_Liu Bio[2]-1.jpg](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2F0vv8moc6%2Fchroma%2Fbdc67a7da979cf6ba6cf989305aae36e6b3a9575-200x262.jpg%3Ffit%3Dcrop%26auto%3Dformat&w=3840&q=75)




