Using Multi-Detection GPC/SEC to Determine Impact of Sterilization on Medical-Grade Polymers
Multi-detection GPC/SEC is described and new data showing how the technique is being used to determine the impact of sterilization on medical grade poly(L-lactide) is presented.
Sterilization is a core part of the medical-grade polymer formulation process. However, high-energy sterilization techniques can have a destructive influence on core polymer features inducing chain scission, bond cleavage, and changes in molecular weight (Mw). For high-end polymers with engineered functionality, such as novel medical materials for timed drug release, the influence of sterilization must be carefully monitored because changes to polymer structure can substantially alter performance. Traditional single detection gel permeation/size-exclusion chromatography (GPC/SEC) is limited in its application for novel material analysis because there are not the appropriate reference standards needed and it does not give the advanced structural analysis required. Multi-detection GPC/SEC overcomes these issues by combining light scattering, refractive index (RI), and viscometry detection to determine absolute Mw and detailed structural information. This article gives an introduction to multi-detection GPC/SEC and presents new data showing how the technique is being used to determine the impact of sterilization on medical grade poly(L-lactide).
Polymer-based controlled drug release systems are used to deliver discrete therapeutic drug dosages to patients over days, weeks, and even months following a single injection. These systems avoid the need for repeated administration, and so decrease patient discomfort and save on healthcare resources. Certain polymer materials can be manipulated to dissolve at selective rates in vivo to ensure that a precise quantity of drug is released at the required time. Such behaviour is tailored to a given specification by precisely engineering performance-defining polymer characteristics — including molecular weight (Mw), molecular weight distribution, and structural features such as branching — during the formulation process.

(PHOTO CREDIT: MIGUEL MALO/GETTY IMAGES)
It is vital to monitor molecular weight parameters throughout the preparatory stages leading up to administration, particularly following sterilization. Energy applied by processes such as gamma irradiation and electron collision sterilization often exceed the level needed to induce polymer bond breakage and chain scission, which means that sterilization can directly affect molecular weight characteristics. Appropriate polymer characterization techniques provide the detailed insight required to quantify these changes.
Multi-Detection GPC/SEC
GPC/SEC enables the determination of molecular weight for polymers, proteins, and macromolecules. The first stage of the analysis involves the separation of a dissolved sample on the basis of size using a column containing microporous packing material. The second step involves detection of eluting compounds, and this determines the amount of information generated.
Traditional GPC/SEC techniques incorporate a single refractive index (RI) detector that determines the concentration of samples in each size fraction and produces a relative molecular weight distribution via a calibration curve. It relies on external reference standards for calibration because an RI detector, in isolation, is unable to measure absolute molecular weight. In addition, single detection GPC/SEC offers no information about the material structural characteristics. For custom-engineered polymers without easily assessable reference standards, and where structure strongly influences functionality, single detection GPC/SEC cannot provide the data needed to support development.

Figure 1: (a) Smaller, isotropic scattering molecules scatter light evenly in all directions while (b) anisotropic scatterers scatter light at different angles at different intensities.
Modern GPC/SEC instruments often incorporate additional detectors alongside RI to increase the information flow from each experiment. For example, triple detection GPC/SEC also includes a light scattering detector and a viscometer. A light-scattering detector uses the direct relationship between the angle and intensity at which molecules scatter light, and the molecular mass to calculate absolute molecular weight data without any requirement for column calibration. A viscometer determines intrinsic viscosity (IV) from measurements of the pressure drop across a capillary bridge as a function of sample solution flow rate. IV is an important molecular characteristic directly related to the density of the macromolecules and is essential in determining accurate structural information.
These additional detectors give valuable information when used individually but also work in tandem to maximize analytical insight. For example, absolute molecular weight data derived from light scattering can be used alongside IV measurements to investigate the structural characteristics of a polymer by using a Mark-Houwink (MH) plot. An MH plot is a log–log graph of intrinsic viscosity against molecular weight distribution and its y intercept, gradient, and overall shape yield information. For example, a material with a high degree of branching is likely to have a shallower slope than one with a more linear structure. The ability of multi-detection GPC/SEC to deliver this information makes it a valuable technique for medical polymer analysis and development.
Case Study: Using Multi-Detection to Monitor Structural Changes Following Sterilization
A study was performed to investigate the influence of high-energy sterilization on three medical-grade, commercially available block and co-block polylactic acid (PLA)-based polymers. These materials are biocompatible in vivo and dissolve within the body at a rate determined by molecular weight and structural features of the polymer. Molecular weight and structure can be controlled by varying reaction conditions to deliver the flexibility required for timed-release systems.
Method: Three commercially available PLA copolymers, two from Corbian Purac (Purasorb PDLG 7502A and Purasorb PLDL 7028A [PDL]) and one from PolySciTech (PolyVivo-AK24) were analyzed using a triple detection GPC/SEC system (Viscotek TDAmax, Malvern Instruments), prior to and following either electron collision sterilization (e) or gamma irradiation (g).Both kinds of samples (gamma and electron sterilized) were exposed to an absorbed radiation level of 25 kGy (25000 Grays) for a few seconds. The GPC/SEC system consisted of a light scattering detector (LALS+RALS), viscometer, and RI detector. Samples were dissolved in chloroform and gently agitated to fully dissolve samples and ensure homogeneity prior to injection onto the system. Table 1 summarizes the results obtained for each sample where Mw is the average molecular weight (Da), Mn is number average molecular weight (Da), Mw/Mn is polydispersity, IV is intrinsic viscosity (dL/g), and Rh is hydrodynamic radius (nm).
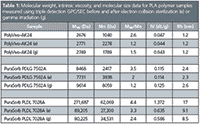
Table 1: Molecular weight, intrinsic viscosity, and molecular size data for PLA polymer samples measured using triple detection GPC/SEC before and after electron collision sterilization (e) or gamma irradiation (g).
Results: One initial observation from the results was that the reported Rh values span the boundary of isotropic and anisotropic scattering (see box "Light Scattering Explained"), a transition which occurs at around 10–15 nm radius. In this instance successful analysis of the samples was therefore enabled by the coupling of both RALS and LALS detectors, which allows the analysis of relatively small and large particles within the same experiment.

Light Scattering Explained - LALS, RALS, and MALS
Of the three polymer samples, only PLDL underwent substantial physical changes following sterilization. The Mw significantly decreased following both sterilization treatments, a change that can be clearly seen from the shift to the left in the molecular weight distribution plot (Figure 2). The calculated polydispersity is appreciably lower for the treated samples. This marked change in physical characteristics suggests that sterilization has a destructive influence on the polymer structure and may potentially impact product performance.

Figure 2: Molecular weight distribution overlay of PuraSorb PLDL 7028A before and after sterilization treatment. Green = before treatment. Orange = gamma irradiation. Purple = electron collision.
A Mark-Houwink plot (Figure 3) for the untreated PLDL (blue) and for each treated PLDL sample (red and green) illustrates how the structural characteristics of the polymer have been altered by sterilization. The plots for the treated PLDL samples differ clearly from the untreated sample, particularly within the high Mw region. Here the slope is noticeably shallower for the treated samples, suggesting that these polymers are denser. Increases in density are associated with a less linear structure and a greater degree of branching.
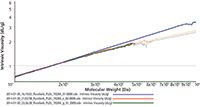
Figure 3: A Mark-Houwink plot for treated vs. untreated PuraSorb PLDL 7028A samples shows that the degree of branching within the higher molecular weight region is changed by sterilization.
However, the plots do not exhibit such a pronounced difference in gradient in the low molecular weight region or vary in terms of their point of intercept, indicating that the specific monomeric density (and hence the structure of the repeating unit) and the uniformity of low molecular units is unaffected by sterilization. One interpretation of this data is that the degradation process has broken down the polyuniformity of the polymer into smaller fragments, increasing internal cross-branching without affecting the monomeric structure. These changes, along with the striking decrease in Mw, suggest that high energy gamma irradiation and electron collision sterilization disrupt the PLDL structure and may impact performance. Although it is hard to directly correlate how sterilization will influence drug release, these results clearly suggest that the PolyVivo-AK24 and Purasorb PDLG 7502A, which are unaffected by sterilization, may be more suitable for this application.
Conclusion
Materials with built-in functionality help to deliver closely controlled drug delivery performance but their development relies on smart analytical techniques that offer the insight required to understand and shape polymer performance. Multi-detection GPC/SEC combines light-scattering, refractive index, and viscometer detection to provide sophisticated polymer and macromolecular characterization beyond what is achievable with conventional single detection techniques. The results presented here illustrate how multi-detection GPC/SEC enables quantification of the destructive influence of sterilization on medical-grade polymers and supports the development of effective preparation methods.
Bassem Sabagh is a product technical specialist at Malvern Instruments, UK, and is an expert on the application of GPC/SEC for the characterization of natural and synthetic polymers and macromolecules. He has been at Malvern Instruments since 2008 supporting customers in their use of company products.
Dr. Angel Valero-Navarro is a founder and COO (Chief Operating Officer) of the technology-based company Nanomateriales y Polimeros (nanoMyP) in Armilla, Spain. Angel's research interests lie predominantly in the areas of synthetic polymer chemistry and materials science, with special emphasis on the design and the synthesis of functional polymeric nanomaterials.
E-mail: salesinfo@malvern.com
Website: www.malvern.com
This article is from The Column. The full issue can be found here: http://images2.advanstar.com/PixelMags/lctc/digitaledition/June05-2014-uk.html

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










