Using Benzoyl Chloride Derivatization to Improve Small-Molecule Analysis in Biological Samples by LC–MS/MS
LCGC North America
In metabolomics, LC–MS is a popular technique because of its ability to separate a wide range of metabolites, but the presence of many highly polar analytes in these samples poses a challenge. Benzoyl chloride derivatization can be a practical solution, improving both sensitivity and selectivity.
In this article, we describe the process of sample preparation and method development for the analysis of polar small molecules using benzoyl chloride (BzCl) derivatization and liquid chromatography–tandem mass spectrometry (LC–MS/MS). We also highlight several recent applications of these techniques to metabolomics analyses.
Metabolomics, or the measurement of small molecules in biological systems, is unique compared to other "omics" disciplines because it focuses on the expressed phenotypes at the end of molecular pathways. This approach enables the comparison of both normal and abnormal biological processes and the molecular responses to a wide variety of external stimuli (1–3). Many different application areas can be studied through the use of metabolomic techniques. Monitoring small-molecule neurotransmitters and studying neurotransmission within neural circuits and networks is important for determining pharmacological effects and parsing behavioral responses within the brain (4). The identification of metabolites that act as biomarkers and change in response to different disease states can also be important for clinical analysis (5,6). This approach can be further expanded into using biomarkers to determine specific drug target sites, resulting in metabolomics playing a key role throughout the pharmaceutical discovery process (7). A number of other application areas have also been studied by these methods, including microbiology, plant biology, animal physiology, food science, and more (8).
In all of these different fields, metabolomic samples can be difficult to analyze because of their complex variety of chemical functionalities and wide range of concentrations (9). To help resolve these issues, many analytical separation techniques, usually coupled to mass spectrometry (MS) detection, have been applied to metabolomics samples (10). MS detection is desirable because of its high sensitivity, wide dynamic range, and high selectivity, which are important for assaying the large number of analytes with varying concentrations in a typical biological sample. Gas chromatography–mass spectrometry (GC–MS) is a highly efficient separation technique, but lengthy sample derivatization processes are often required to ensure analyte volatility (11). Capillary electrophoresis–mass spectrometry (CE–MS) enables the injection of very small (nanoliter) volumes and is good for the separation of polar ionic species, but the buffers needed to improve the separation of neutral molecules (that is, micellar electrokinetic chromatography [MEKC]) are not compatible with electrospray ionization (12). Liquid chromatography–mass spectrometry (LC–MS) provides separation capabilities for a wider range of metabolites compared to the other two methods, leading to its popularity for metabolomics analysis over the past several years (13).
Despite its strengths, metabolomics method development using LC can be challenging because many metabolites are highly polar and exhibit little to no retention with the hydrophobic stationary phases found in reversed-phase columns. This lack of retention is particularly problematic for LC–MS analysis because the elution of polar compounds in the void volume coincides with the elution of many of the salts present in the injected sample, causing matrix interferences that reduce ionization efficiency and MS signal (14). One way to enhance the retention of polar analytes is through the use of hydrophilic-interaction chromatography (HILIC) columns; however, the more hydrophobic analytes in the mixture then are eluted early and the same issues occur. Another method is to derivatize analytes with a nonpolar group, which provides increased retention to all derivatized analytes in reversed-phase LC and enhances the ionization efficiency of some analytes for MS analysis (15). A recent review compared six different derivatizing agents used for this purpose of increasing the hydrophobicity of polar analytes in biological samples (16). Of these six reagents, benzoyl chloride (BzCl) demonstrates significantly faster reaction times in milder conditions, making it the preferred derivatization technique for routine analysis of metabolites by LC–MS. In this article, we describe the process of sample preparation and method development for the analysis of polar small molecules using BzCl derivatization and LC–MS/MS, and highlight several recent applications of these techniques to metabolomic analyses.
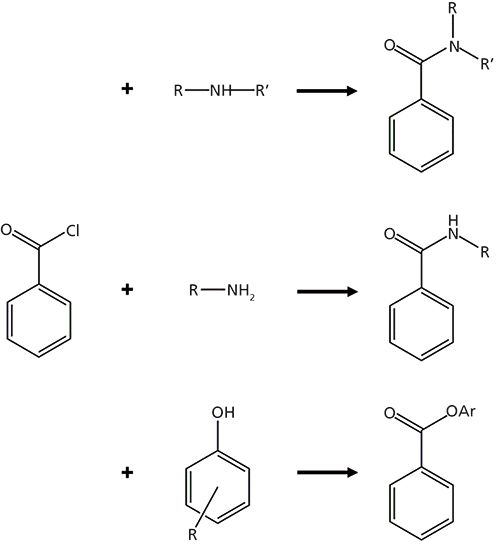
Figure 1: Reaction scheme detailing the derivatization of primary amines, secondary amines, and phenols with benzoyl chloride. Adapted with permission from reference 18. Copyright American Chemical Society (2012).
Benzoyl Chloride Derivatization Methods
Labeling by BzCl follows the Schotten-Baumann reaction scheme used for the esterification or amidation of acid chlorides (17) and provides a number of desirable characteristics as a derivatizing agent for polar metabolites. It reacts with a wide variety of functional groups, including primary and secondary amines and phenols (similar to dansyl chloride), as well as ribose-hydroxyl groups (Figure 1) (18). Benzoylation is an ideal derivatization reaction because it is fast (typically requiring less than 1 min for sample preparation), can be performed at room temperature, and generates products that are stable (for up to six months at -80 °C) (19). The added benzoyl groups increase the retention of polar analytes and are easily fragmented during MS/MS analysis, improving both the separation and detection aspects of the method. Analyte signal is also enhanced by improved ionization efficiency of derivatized analytes, likely because of the increased hydrophobicity aiding in charge formation during droplet desolvation, as has also been observed with dansyl chloride derivatization (20). Comparing the extracted ion chromatograms of native and benzoylated neurotransmitter dopamine (DA) in Figure 2, the derivatized compound is retained 4.6 times longer and has a peak height more than 100 times larger. Finally, a heavy isotope 13C6-BzCl derivatizing agent is commercially available at relatively low costs, providing an opportunity to generate a unique internal standard for every analyte of interest. The use of these internal standards can help improve both quantitation and method repeatability for LC–MS/MS analysis.
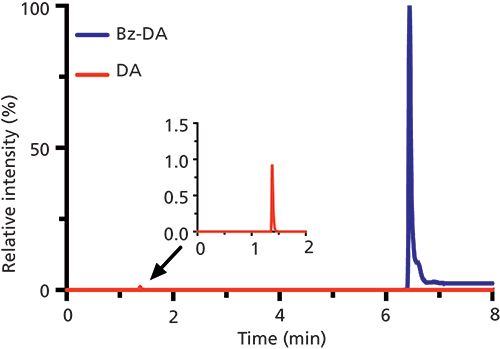
Figure 2: Comparison of LC–MS/MS signal for dopamine (DA) and derivatized dopamine (Bz-DA). Data provided courtesy of Paige Malec and Robert Kennedy (University of Michigan).
The general workflow for BzCl derivatization of a sample is shown in Figure 3. For benzoylation to occur, the sample must be at a pH of at least 9. Originally, an addition of sodium tetraborate buffer was used to achieve basicity, but more recent studies have found that sodium carbonate provides more-sensitive detection for many analytes, now making it the preferred reagent for this step (19). After the pH has increased and stabilized, an aliquot of BzCl in acetonitrile (usually 2% v/v) is added for derivatization. This concentration ensures solubility of BzCl in the solvent while also providing an excess of the derivatizing agent to make certain that the reaction goes to completion. Then, a final acidic internal standard reagent containing previously prepared 13C6-Bz-labeled internal standards is added to quench the reaction. Both formic acid and sulfuric acid have been used to decrease the pH and enhance sample stability, but sulfuric acid provides a higher signal for some compounds, potentially because of a reduction in formate adducts (18). Initially, the method used dimethyl sulfoxide (DMSO) as a solvent for this solution, but the high organic content led to poor peak shapes for early eluted compounds. More recently, 20–50% acetonitrile in water has been used, which limits the total organic content of the injection solvent (combining the dialysate fraction and all three reagent solutions) to about 30% and improves band focusing at the head of the column. In general, for every 1 µL of sample that is to be derivatized, 0.5 µL of each of these three reagents is added. Other ratios can be used depending on the injection volume desired for LC, as long as sufficient BzCl is added to label all analytes and calibration standards are treated in the same manner.
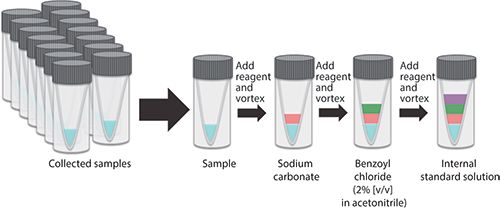
Figure 3: Schematic demonstrating the workflow for derivatizing biological samples with benzoyl chloride.
LC–MS/MS of Benzoylated Analytes in Targeted Methods
For specific applications, targeted LC–MS/MS metabolomics techniques focused on quantitating a subset of analytes related to a particular molecular pathway can be used (21). By using a triple-quadrupole (QqQ) mass spectrometer for multiple reaction monitoring (MRM), relevant mass transitions can be used to ensure high selectivity during analysis, with analyte identification achieved by LC retention time, parent mass-to-charge ratio, and fragmentation pattern. Every metabolite of interest will have a unique precursor mass (based on the analyte molecular weight and the number of added benzoyl groups equal to the number of derivatization sites) and a product mass related to the cleavage of the derivatization group. Usually, this product mass will either have an m/z value of 105 or 111 based on whether it is an analyte or internal standard, respectively. With the use of 13C6-Bz-labeled internal standards added for each measured metabolite, the number of components that must be separated and detected by LC–MS/MS is doubled. Even though MS detection is selective enough to allow for peak overlaps, extensive coelutions should be avoided with high peak capacity separations to prevent ionization interference between analytes and to ensure a sufficient number of mass spectra are acquired for each peak. In addition to tandem-MS methods, high-resolution MS techniques can also be used for analyte identification based on mass shifts that occur because of derivatization (22).
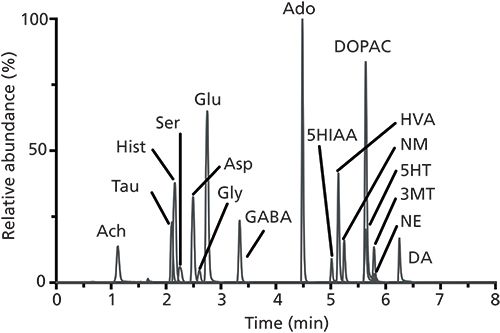
Figure 4: Overlaid extracted ion chromatograms from the analysis of 17 neurotransmitters and metabolites (with abbreviations defined in the original article) by LC–MS/MS. Adapted with permission from reference 18. Copyright American Chemical Society (2012).
The Kennedy laboratory reported one of the first targeted metabolomics methods for multiple analytes using BzCl derivatization and LC–MS/MS with the separation of 17 neurochemicals from a dialysate sample in 8 min (Figure 4) (18). A more comprehensive 70-compound method taking 20 min has also been developed (Figure 5), with the goal of demonstrating benzoyl chloride derivatization as a key component in widely targeted analytical assays (19). The value of monitoring a larger number of analytes is the ability to study interactions throughout entire metabolic pathways, providing a better understanding of changes that occur in these processes under certain conditions or potentially uncovering previously unknown relationships between different analytes. If a smaller number of metabolites is of particular interest to test a specific hypothesis, throughput can be improved by increasing separation speed. For example, extracted ion chromatograms from a 2-min separation of 24 neurotransmitters are shown in Figure 6 (23). By using columns packed with sub-2-µm superficially porous particles and ultrahigh-pressure liquid chromatography (UHPLC) instrumentation capable of pressures up to 1500 bar, higher flow rates for these fast separations can be achieved while still maintaining sufficient chromatographic efficiency to avoid peak overlaps. These fast LC–MS/MS assays are most important when extensive studies generating hundreds or thousands of samples in a single experiment are conducted. By increasing the analytical throughput 3–4 times with faster separations, the total experimental time can be reduced dramatically.
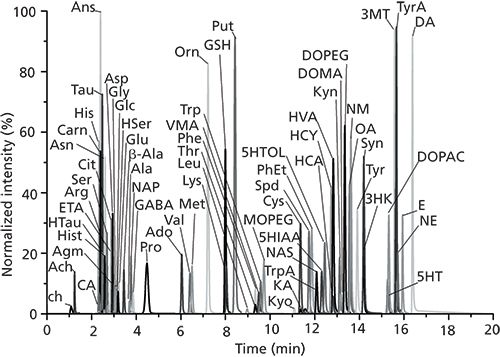
Figure 5: Overlaid extracted ion chromatograms from the analysis of 70 metabolites (with abbreviations defined in the original article) by LC–MS/MS. Adapted with permission from reference 19.
The Use of Benzoyl Chloride Derivatization and LC–MS/MS in Neuroscience Applications
Most of the initial demonstrations of BzCl derivatization of biological samples followed by LC–MS/MS analysis have focused on the study of neurotransmitters collected by microdialysis. Microdialysis, in which dialysate is collected from the brain through a semipermeable membrane, is an unbiased sampling technique used for the collection of essentially all compounds below the membrane's molecular weight cutoff (24). Widely targeted assays can reduce the sample volume required for microdialysis collection, because a single fraction can be used to simultaneously measure many more neurotransmitters than is possible with other methods. Decreasing the collected volume helps increase the temporal resolution of the technique, which improves the observation of chemical events at faster timescales. Since the initial report of BzCl derivatization as a way to improve LC–MS/MS analysis of neurotransmitters, a number of different application areas have been explored, such as pharmacological and drug response as well as behavioral studies. Let's take a closer look at those application areas.
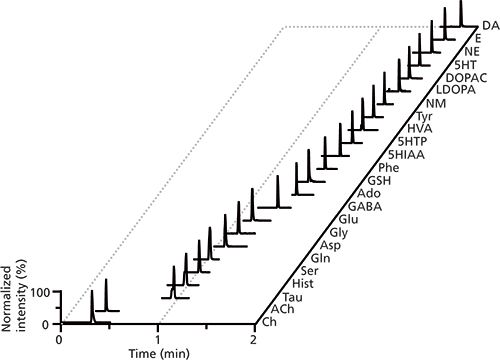
Figure 6: Extracted ion chromatograms from the analysis of 24 neurotransmitters and metabolites (with abbreviations defined in the original article) by LC–MS/MS. Adapted with permission from reference 23.
Pharmacological and Drug Response
Identifying and understanding different metabolic pathways in brain circuits is important in the research and development of pharmacological compounds intended to alter these pathways for a desired therapeutic response. This analysis can be achieved by measuring relative changes in metabolite concentrations over time with microdialysis as certain pharmacological stimuli are applied. More specifically, microdialysis can be used to track neurotransmission during illicit drug use to help understand how addiction forms and to determine therapeutic targets. Most drugs of abuse increase dopamine (DA) release in the reward center of the brain and induce hyperlocomotion (that is, increased movement activity) in rodents (25). Understanding the relationship between locomotor effects and how these drugs of abuse alter neurochemical activity is necessary for the development of potential treatments (26). Further studies using in vivo microdialysis and simultaneous locomotor behavior monitoring to analyze potential inhibitors of the amphetamine mechanism found that PKCβ inhibitors reduced amphetamine stimulated locomotion and DA release without altering basal levels of DA or other neurotransmitters (27). The temporal resolution of this LC–MS/MS method (3-min fractions) provided better correlation of the observed behavior with neurochemical concentrations during drug use than if longer time fractions (and thus, larger volume) had been needed for multiple assays.
Most studies use standard-size microdialysis probes to collect dialysate for analysis. To improve the spatial resolution of these measurements, microfabricated microdialysis probes can be used to assess discrete brain regions as small as 70 µm and reduce probe-related tissue damage by 80% (28). In addition to their small size, these miniature silicon fabricated probes are sturdy enough to be implanted directly into the brain without additional support. In a proof-of-principle study, these miniature probes were implanted in anesthetized animals in vivo to demonstrate that amphetamine stimulated changes in neurotransmitters were comparable to the larger probes as measured by LC–MS/MS following derivatization of the samples with BzCl. Although these results are promising, future tests in awake, freely moving animals should be performed and the cost and feasibility of scaling up must be assessed.
Behavioral Studies
A key advantage of microdialysis is that it can be used for sample collection in awake, moving animals, which allows for the identification of multiple, specific neurotransmitters involved in both normal and pathophysiological behaviors. For example, 17 striatal neurotransmitters were examined in a mouse model of the movement disorder dystonia, and only acetylcholine (ACh) showed significant decrease compared to a control mouse (29). After this initial multianalyte assay, a follow-up experiment using LC–MS/MS with higher specificity for ACh allowed the researchers to focus on cholinergic function and correlate this decrease to movement abnormalities (29). In another study, the BzCl assay was used to demonstrate that dopamine release in the nucleus accumbens reflects a motivational signal at 1-min timescales (30). BzCl derivatization was coupled with 1-min microdialysis fraction collections to study 26 neurochemicals and showed extracellular DA concentration changes during trial-and-error choice tasks correlated with reward rate, a metric for the value of engaging work. Further research on this topic using BzCl demonstrated that these value signals are localized to specific subregions of the striatum and cortex, suggesting that DA release is not uniform throughout all interconnected regions (31).
The regulation of feeding behavior has also been investigated using BzCl derivatization. Many reports suggest that neurochemical differences are associated with obesity and increased body mass index (BMI) (32). A BzCl study performed in rats prone to obesity found that these animals displayed increased locomotor activity in response to cocaine, which inhibits monoamine transporters. The analysis of nucleus accumbens microdialysis samples by LC–MS/MS following BzCl derivatization revealed no differences in DA, serotonin, nor their metabolites between obesity prone rats and obesity resistant control rats (33). The norepinephrine (NE) pathway was examined next because cocaine also acts on the NE transporter. Desipramine (a more specific NE transporter inhibitor than cocaine) was administered locally through the microdialysis probe directly into the nucleus accumbens to more precisely target the NE pathway. After inhibiting the NE transporter, a significant increase in NE levels was observed only in the obesity resistant rats, indicating a discrepancy in this neurotransmitter system for obesity-prone rats. This finding provides an area to focus on in future investigations exploring the relationship between neurotransmission, feeding, and weight gain (34).
BzCl derivatization has also been combined with recent techniques that provide selective activation or inhibition of specific neuronal populations in vivo. The ability to modulate the activity of neurons on command provides researchers precise tools to understand the underlying functions of neuronal circuits and is even more powerful when correlated with observed changes in neurochemical concentrations. Two techniques for selective stimulation include optogenetics and designer receptors exclusively activated by designer drugs (DREADDs) (35,36). Optogenetics uses light to selectively activate or silence specific neurons, whereas DREADDs are used to activate modified G-protein-coupled receptors by administering otherwise biologically inert compounds. These powerful techniques are often combined with behavioral, pharmacological, and ex vivo studies to further evaluate physiological circuits and disease states. However, direct in vivo measurements of neurochemical dynamics with these selective stimulation techniques have been scarce (37–41). BzCl derivatization was used with DREADDs-mediated selective activation of a specific population of hypothalamic neurons (containing neurotensin peptide) to monitor the release of small molecules within the nucleus accumbens in awake mice (37). This study found that activation of these specific neurons resulted in an efflux of DA and its metabolites in the nucleus accumbens and promoted a DA-dependent locomotor activity. These results confirmed the presence of a neural circuit between these regions (hypothalamus to nucleus accumbens), and revealed an important function in dopamine regulation. A separate optogenetic study coupled with microdialysis in awake mice examined the effect of selective activation of a population of neurons (containing the opioid peptide dynorphin) in subregions of the nucleus accumbens shell separated by 1 mm. Specific regional differences in DA signaling were observed with optogenetic stimulation, which corresponded to specific behavioral phenotypes related to place preference (42).
Other Applications Using LC–MS/MS to Characterize Benzoyl Chloride–Derivatized Samples
In addition to the many studies focused on in vivo neurotransmission, a number of other metabolomic applications have also been explored using BzCl derivatization.
Food Analysis
The measurement of biogenic amines in food samples is important because these products of metabolic processes in bacteria indicate food spoilage and can also cause physiological effects in consumers (43). Since these compounds typically contain primary and secondary amines, BzCl derivatization is helpful in developing assays to measure them. In one report, a 48-min LC–MS/MS method for 14 biogenic amines that included 10 isotope-labeled internal standards was used to measure these compounds in foods that involve bacteria in their production or are susceptible to bacterial spoilage (44). For this study, the internal standards had the stable isotopes included in their core molecular structure rather than on the BzCl label, which required the synthesis of some internal standards that were not available commercially. A more recent application of BzCl derivatization was used for the monitoring of nine bacterial metabolites (with two internal standards) in supermarket fish (45). With a 15-min separation method, the authors demonstrated detection limits on the order of 0.1–10 pg/mL for analytes that affect the taste and smell of fish and potentially cause food poisoning. In both of these examples, the use of 13C6-BzCl could be implemented to simplify the creation of stable isotope–labeled internal standards. This strategy was demonstrated for wine analysis through the development of a 56-compound method, each with its own unique internal standard, with submicromolar detection limits reported for all analytes (46).
Clinical and Biological Pathway Analysis
Because of the wide applicability of BzCl derivatization to biologically relevant compounds, this technique has not only been applied to monitor metabolites in microdialysis samples, but many other matrices including human cerebrospinal fluid (CSF), human plasma, human serum, rat plasma, fly tissue homogenate, and fly hemolymph (19,47). For example, Drosophila tissue homogenate derivatized with BzCl and analyzed with LC–MS/MS found that starved flies contained fivefold increased octopamine (a fly-specific compound analogous to norepinephrine) compared to fed flies (19). Increased octopamine activity with starvation has been linked to foraging-like behaviors in flies as they presumably attempt to locate food (48,49). The serotonin metabolite 5-hydroxyindoleaceticacid (5-HIAA) was also unexpectedly observed in the tissue homogenate (19), despite the assumed lack of monoamine oxidase activity in flies (50). This study is an example of how a comprehensive targeted assay can be beneficial in observing unexpected results that can potentially lead to new areas of investigation.
Precolumn derivatization with BzCl (and other derivatization agents) can be used on human CSF. followed by analysis with LC–MS/MS to provide insight into biomarkers for neurological disease diagnosis and potential pharmacological targets, as well as to identify the effects of pharmaceutical agents in clinical trials (51,52). The method also applies to animal models of specific diseases (53,54). For the clinical analysis of non-CSF samples from humans, techniques using LC–MS/MS of BzCl-derivatized samples were used to determine glucose and glycerol concentrations in plasma to improve sensitivity in in vivo metabolic studies of glucose kinetics (55). A similar method was used to measure glycerol in urine samples as a marker for banned substances in athletes because of its common use as a doping masking agent (56). Other clinical applications used a higher mass accuracy instrument (a quadrupole time-of-flight MS system) for the identification of BzCl-derivatized polyamines that serve as potential cancer biomarkers in human plasma (57) and urine (58) without the need to identify MRM transitions for each compound. Finally, BzCl derivatization was used in the first report of the quantitative detection of the opioid-like small molecule kyotorphin in human serum (19). Kyotorphin is a biomarker candidate for neurodegenerative diseases such as Alzheimer's disease (59) and has been previously detected in CSF samples obtained from lumbar puncture, but less invasive blood sampling to monitor its concentration would be beneficial for patients.
Conclusions and Future Directions
BzCl derivatization and LC–MS/MS have many advantages for developing targeted analyses and can be applied to a wide range of metabolomic applications. Further improvements in LC and MS technology will eventually lead to even better detection limits and increase the number of analytes observed in a single assay. For LC, the use of narrower diameter columns can help improve sensitivity as well as provide the opportunity to use smaller injection volumes, which would be useful for the lower volumes associated with biological samples, especially those collected by microdialysis. Recent reports of long, high-efficiency capillary columns used with a gradient UHPLC instrument capable of achieving 3000 bar have shown the potential improvements that can be made in peak capacity from current commercial systems (60). This system was recently used for the analysis of BzCl-derivatized neurotransmitters and other metabolites to demonstrate how enhanced separation efficiency can improve metabolomics analysis (61). In MS, achieving faster scan rates to ensure that a sufficient number of data points are collected per peak will be necessary as chromatographic peak widths decrease. These higher acquisition rates may be difficult to achieve with some triple-quadrupole instrumentation, so other techniques using time-of-flight analyzers such as parallel reaction monitoring (62) could be useful. However, even with current technology, the many applications of LC–MS/MS analysis of BzCl-derivatized metabolites discussed here clearly show the utility of this technique and its potential to improve both the sensitivity and selectivity of targeted metabolomics studies.
References
(1) D. Vuckovic, Anal. Bioanal. Chem. 403, 1523–1548 (2012).
(2) E.M. Lenz and I.D. Wilson, J. Proteome Res. 6, 443–458 (2007).
(3) J.C. Lindon, E. Holmes, M.E. Bollard, E.G. Stanley, and J.K. Nicholson, Biomarkers 9, 1–31 (2004).
(4) R.T. Kennedy, Curr. Opin. Chem. Biol. 17, 860–867 (2013).
(5) A. Koulman, G.A. Lane, S.J. Harrison, and D.A. Volmer, Anal. Bioanal. Chem. 394, 663–670 (2009).
(6) E. Holmes, I.D. Wilson, and J.K. Nicholson, Cell134, 714–717 (2008).
(7) D.G. Robertson, M.D. Reily, and J.D. Baker, J. Proteome Res. 6, 526–539 (2007).
(8) S.P. Putri, Y. Nakayama, F. Matsuda, T. Uchikata, S. Kobayashi, A. Matsubara, and E. Fukusaki, J. Biosci. Bioeng. 115, 579–589 (2013).
(9) H.G. Gika, I.D. Wilson, and G.A. Theodoridis, J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 966, 1–6 (2014).
(10) A. Zhang, H. Sun, P. Wang, Y. Han, and X. Wang, Analyst 137, 293–300 (2012).
(11) H. Kanani, P.K. Chrysanthopoulos, and M.I. Klapa, J. Chromatogr. B871, 191–201 (2008).
(12) M.R.N. Monton and T. Soga, J. Chromatogr. A 1168, 237–246 (2007).
(13) B. Zhou, J.F. Xiao, L. Tuli, and H.W. Ressom, Mol. BioSyst. 8, 470–481 (2012).
(14) N.L. Kuehnbaum and P. Britz-McKibbin, Chem. Rev. 113, 2437–2468 (2013).
(15) O.S. Barnaby, Y. Benitex, J.L. Cantone, C.A. McNaney, T. V Olah, and D.M. Drexler, Bioanalysis 7, 2501–2513 (2015).
(16) A.G. Zestos and R.T. Kennedy, AAPS J. in press (2017). DOI: 10.1208/s12248-017-0114-4
(17) J.J. Li, in Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications (Springer Berlin Heidelberg, Berlin, Heidelberg, Germany, 2003), pp. 362–362.
(18) P. Song, O.S. Mabrouk, N.D. Hershey, and R.T. Kennedy, Anal. Chem. 84, 412–419 (2012).
(19) J.-M.T. Wong, P.A. Malec, O.S. Mabrouk, J. Ro, M. Dus, and R.T. Kennedy, J. Chromatogr. A 1446, 78–90 (2016).
(20) K. Guo and L. Li, Anal. Chem. 81, 3919–3932 (2009).
(21) W. Lu, B.D. Bennett, and J.D. Rabinowitz, J. Chromatogr. B 871, 236–242 (2008).
(22) R. Zhou, C.-L. Tseng, T. Huan, and L. Li, Anal. Chem. 86, 4675–4679 (2014).
(23) J.P. Grinias, J.-M.T. Wong, and R.T. Kennedy, J. Chromatogr. A 1461, 42–50 (2016).
(24) K.N. Schultz and R.T. Kennedy, Annu. Rev. Anal. Chem. 1, 627–661 (2008).
(25) G. Di Chiara and A. Imperato, Proc. Natl. Acad. Sci. U.S.A. 85, 5274–5278 (1988).
(26) O.S. Mabrouk, D.Z. Semaan, S. Mikelman, M.E. Gnegy, and R.T. Kennedy, J. Neurochem. 128, 152–161 (2014).
(27) A.G. Zestos, S.R. Mikelman, R.T. Kennedy, and M.E. Gnegy, ACS Chem. Neurosci. 7, 757–766 (2016).
(28) W.H. Lee, T. Ngernsutivorakul, O.S. Mabrouk, J.-M.T. Wong, C.E. Dugan, S.S. Pappas, H.J. Yoon, and R.T. Kennedy, Anal. Chem. 88, 1230–1237 (2016).
(29) S.S. Pappas, K. Darr, S.M. Holley, C. Cepeda, O.S. Mabrouk, J.-M.T. Wong, T.M. LeWitt, R. Paudel, H. Houlden, R.T. Kennedy, M.S. Levine, and W.T. Dauer, Elife 4, e08352 (2015).
(30) A.A. Hamid, J.R. Pettibone, O.S. Mabrouk, V.L. Hetrick, R. Schmidt, C.M. Vander Weele, R.T. Kennedy, B.J. Aragona, and J.D. Berke, Nat. Neurosci. 19, 117–126 (2015).
(31) J.R. Pettibone, "Valuation and Decision-Making in Cortical-Striatal Circuits," thesis, University of Michigan, 2016.
(32) K.E. Demos, T.F. Heatherton, and W.M. Kelley, J. Neurosci. 32, 5549–5552 (2012).
(33) P.J. Vollbrecht, O.S. Mabrouk, A.D. Nelson, R.T. Kennedy, and C.R. Ferrario, Obesity 24, 670–677 (2016).
(34) K.M. Nesbitt, C.R. Ferrario, and R.T. Kennedy, "In Vivo Microdialysis Calibration and Neurochemical Measurement Using LC–MS/MS in an Animal Model of Obesity," presented at HPLC 2016, San Francisco, California, 2016.
(35) B.N. Armbruster, X. Li, M.H. Pausch, S. Herlitze, and B.L. Roth, Proc. Natl. Acad. Sci. U. S. A. 104, 5163–8 (2007).
(36) K. Deisseroth, Nat. Methods8, 26–29 (2011).
(37) C.M. Patterson, J.-M.T. Wong, G.M. Leinninger, M.B. Allison, O.S. Mabrouk, C.L. Kasper, I.E. Gonzalez, A. Mackenzie, J.C. Jones, R.T. Kennedy, and M.G. Myers, Endocrinology156, 1692–1700 (2015).
(38) P. Anikeeva, A.S. Andalman, I. Witten, M. Warden, I. Goshen, L. Grosenick, L.A. Gunaydin, L.M. Frank, and K. Deisseroth, Nat. Neurosci. 15, 163–170 (2011).
(39) M. Michaelides, S.A.R. Anderson, M. Ananth, D. Smirnov, P.K. Thanos, J.F. Neumaier, G.-J. Wang, N.D. Volkow, and Y.L. Hurd, J. Clin. Invest. 123, 5342–5350 (2013).
(40) S. Royer, B.V. Zemelman, M. Barbic, A. Losonczy, G. Buzsáki, and J.C. Magee, Eur. J. Neurosci. 31, 2279–2291 (2010).
(41) H. Taylor, J.T. Schmiedt, N. Çarçak, F. Onat, G. Di Giovanni, R. Lambert, N. Leresche, V. Crunelli, and F. David, J. Neurosci. Methods235, 83–91 (2014).
(42) J.-M.T. Wong, "Liquid Chromatography-Mass Spectrometry Strategies for In Vivo Neurochemical Monitoring with Microdialysis," thesis, University of Michigan, 2016.
(43) A. Önal, Food Chem. 103, 1475–1486 (2007).
(44) C.M. Mayr and P. Schieberle, J. Agric. Food Chem. 60, 3026–3032 (2012).
(45) Y. Fu, Z. Zhou, Y. Li, X. Lu, C. Zhao, and G. Xu, J. Chromatogr. A 1465, 30–37 (2016).
(46) P.A. Malec, M. Oteri, V. Inferrera, F. Cacciola, L. Mondello, and R.T. Kennedy, J. Chromatogr. A, in press (2017). DOI: 10.1016/j.chroma.2017.07.061
(47) X. Zheng, A. Kang, C. Dai, Y. Liang, T. Xie, L. Xie, Y. Peng, G. Wang, and H. Hao, Anal. Chem. 84, 10044–10051 (2012).
(48) Z. Yang, Y. Yu, V. Zhang, Y. Tian, W. Qi, and L. Wang, Proc. Natl. Acad. Sci. U. S. A. 112, 5219–24 (2015).
(49) S.J. Sigrist and T.F.M. Andlauer, Nat. Neurosci. 14, 124–126 (2011).
(50) T.L. Paxon, P.R. Powell, H.G. Lee, K.A. Han, and A.G. Ewing, Anal. Chem. 77, 5349–5355 (2005).
(51) J.M. Cox, J.P. Butler, B.S. Lutzke, B.A. Jones, J.E. Buckholz, R. Biondolillo, J.A. Talbot, E. Chernet, K.A. Svensson, and B.L. Ackermann, Bioanalysis7, 2461–2475 (2015).
(52) Y. Benitex, C.A. McNaney, D. Luchetti, E. Schaeffer, T. V. Olah, D.G. Morgan, and D.M. Drexler, Rapid Commun. Mass Spectrom. 27, 1882–1886 (2013).
(53) A. Kovac, Z. Somikova, N. Zilka, and M. Novak, Talanta 119, 284–290 (2014).
(54) D. Li, O.S. Mabrouk, T. Liu, F. Tian, G. Xu, S. Rengifo, S.J. Choi, A. Mathur, C.P. Crooks, R.T. Kennedy, M.M. Wang, H. Ghanbari, and J. Borjigin, Proc. Natl. Acad. Sci. U. S. A. 112, E2073–82 (2015).
(55) A. Bornø, L. Foged, and G. van Hall, J. Mass Spectrom. 49, 980–988 (2014).
(56) Y. Dong, Y. Ma, K. Yan, L. Shen, X. Wang, Y. Xu, G. He, Y. Wu, J. Lu, Z. Yang, and F. Feng, J. Chromatogr. B 957, 30–35 (2014).
(57) R. Liu, K. Bi, Y. Jia, Q. Wang, R. Yin, and Q. Li, J. Mass Spectrom. 47, 1341–1346 (2012).
(58) R. Liu, Y. Jia, W. Cheng, J. Ling, L. Liu, K. Bi, and Q. Li, Talanta83, 751–756 (2011).
(59) M.M.B. Ribeiro, A.R.T. Pinto, M.M. Domingues, I. Serrano, M. Heras, E.R. Bardaji, I. Tavares, and M.A. Castanho, Mol. Pharm. 8, 1929–1940 (2011).
(60) K.M. Grinias, J.M. Godinho, E.G. Franklin, J.T. Stobaugh, and J.W. Jorgenson, J. Chromatogr. A 1469, 60–67 (2016).
(61) B. Wang, J. Felton, P. Malec, S. Moore, J. Treadway, D. Lunn, J. Jorgenson, and R. Kennedy, "Developing an Ultra Ultra High Pressure Liquid Chromatography (UUHPLC) System for LC-MS based Metabolomics," presented at HPLC 2016, San Francisco, California, 2016.
(62) A.C. Peterson, J.D. Russell, D.J. Bailey, M.S. Westphall, and J.J. Coon, Mol. Cell. Proteomics11, 1475–88 (2012).
James P. Grinias is with the Department of Chemistry and Biochemistry at Rowan University in Glassboro, New Jersey. Jenny-Marie T. Wong is with HPLC Product Marketing at Thermo Fisher Scientific in Waltham, Massachusetts. Kathryn M. Nesbitt is with the Department of Chemistry at Towson University in Towson, Maryland. Direct correspondence to: grinias@rowan.edu

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.











