The Use of Liquid Chromatography with Tandem Mass Spectrometry for the Analysis of Emerging Environmental Contaminants
Special Issues
Liquid chromatography with tandem mass spectrometry (LC–MS-MS) led to a revolution in environmental testing. The coupling of liquid chromatography with tandem mass spectrometry created a powerful analytical tool for the analysis of emerging environmental contaminants. Pharmaceuticals and personal care products, perfluorinated compounds, brominated flame retardants, and disinfection byproducts were chosen as examples to illustrate the use of this new technique in environmental analysis.
Traditionally, environmental scientists focused on the analysis of more "classical" environmental pollutants such as dioxins, polychlorinated biphenyls, and chlorinated pesticides (such as DDT). Gas chromatography with mass spectrometry (GC–MS) typically is used to identify and quantify these persistent organic compounds.
However, many organic chemicals are difficult to analyze using traditional GC–MS. Time-consuming and extensive sample preparation is required to reach required sensitivity levels. Liquid chromatography with tandem mass spectrometry (LC–MS-MS) led to a revolution in environmental research over the last decade. LC–MS-MS allows the identification and quantitation of highly polar organic compounds down to nanogram-per-liter levels without time-consuming derivatization and with minimal sample cleanup.
Many emerging environmental contaminants enter the environment after domestic use and are not removed completely by wastewater treatment. Environmental pollutants studied by LC–MS-MS in water and other environmental samples include pharmaceuticals and personal care products (PPCP) such as painkillers, antibiotics, antidiabetics, antidepressants, contraceptives, and lipid regulators. Hormones and other compounds with estrogenic effects and antibiotics are especially a major concern due to a possible development of bacterial resistance. LC–MS-MS is also the analytical technique of choice for the analysis of perfluorinated compounds (PFC), brominated flame retardants (BFR), and drinking water disinfection byproducts (DBP), including haloacetic acids and bromate (1–4).
LC–MS-MS Technology
An LC system with a pump, an autosampler, a column, and a column oven is used in front of the mass spectrometer. Mostly reversed-phase columns with gradients of water, acetonitrile, and methanol with volatile buffers of ammonium formate and formic acid are used for LC–MS-MS to separate analytes of interest and to separate analytes from sample matrix components. After separation, the eluent enters the ion source of the mass spectrometer.
Electrospray ionization (ESI) is the most popular ionization technique. Other LC–MS-MS ion sources are atmospheric pressure chemical ionization (APCI) and atmospheric pressure photo ionization (APPI), which can be used to detect less polar chemicals. In ESI, the eluent is sprayed through a capillary while applying a high voltage to the tip of the spray needle. This procedure generates micrometer-sized droplets containing positively and negatively charged analyte ions. Now gentle heating is used to evaporate solvents and release ions from the droplets. Finally, ions carrying positive or negative charge — depending upon the polarity of the mass spectrometer — are guided by electrical fields into the mass analyzer region. In comparison to traditional GC–MS ionization techniques, such as electron impact (EI), ESI is a very soft technique and produces mostly protonated or deprotonated molecular ions.
Different mass analyzers are used in LC–MS-MS laboratories, with triple-quadrupole systems being used widely. New hybrid technologies, such as triple quadrupole coupled with linear ion trap (QTRAP system, Applied Biosystems, Foster City, California) have been developed recently and are gaining popularity. Triple-quadrupole mass analyzers can be used in many different scan modes with multiple reaction monitoring (MRM) being most popular for quantitation. In MRM mode, the molecular ion is filtered in the first quadrupole (ions of different mass-to-charge ratio [m/z] are not allowed to pass the quadrupole). The filtered molecular ion is then fragmented into product ions by collision with a neutral collision gas (such as nitrogen) in the second quadrupole and the product ion of interest is filtered in the third quadrupole (Figure 1). This double filtering reduces the background noise greatly, allowing quantitation with optimal selectivity and sensitivity, excellent linearity, and reproducibility.
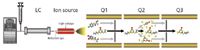
Figure 1: Schematics of LCâMS-MS (LC system, ion source, and triple quadrupole).
Quantitation and Confirmation of PFCs
An example of LC–MS-MS quantitation using MRM for the analysis of PFCs (perfluorooctanesulfonate [PFOS] and perfluorooctanoic acid [PFOA]) is shown in Figure 2. The high selectivity of MRM allows low limits of detection and also the reduction of LC run times, especially with high-resolution LC systems and small particle columns. The chromatogram presented in Figure 2 was generated on a C18 column with 1.8-μm particles and a mobile phase of water–acetonitrile with a buffer of ammonium acetate. PFOS and PFOA were detected on an API 4000 LC–MS-MS system (Applied Biosystems) with ESI in negative polarity, allowing detection limits in the low nanogram-per-liter range by direct injection of water samples. Such methods are in routine use for the analysis of PFCs in various environmental matrices, including water, arctic ice, sediment, fish, and polar bear tissue.
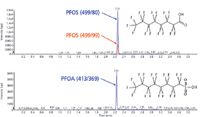
Figure 2: PFOS and PFOA analyzed by LCâMS-MS using ESI in negative polarity and detection in multiple reaction monitoring.
Also, Figure 2 shows an example in which the MRM mode is used to confirm quantitative data by MRM ratio calculation. For PFOS, two MRM transitions were detected (499/80 and 499/99). The ratio of quantifier and qualifier transition is calculated for each sample and compared to the ratio of standard injections. If the ratio of the unknown sample is similar to the ratio of the standard, the quantitative data are confirmed.
Screening and Confirmation of PPCPs
Another application of LC–MS-MS technology is the screening and confirmation for PPCP. For this application, powerful and fast screening capabilities are required, allowing the detection of dozens or even hundreds of targeted analytes in a single injection at very low concentrations in water or other environmental samples. The method developed by Borton and colleagues (5) screens for approximately 100 substances using a single MRM transition. The 4000 QTRAP LC–MS-MS system (Applied Biosystems) was used with ESI in positive and negative polarity after separation on a traditional C18 column with gradient of water, formic acid, and acetonitrile.
In addition, the mass spectrometer automatically acquired full scan and high-sensitivity MS-MS spectra when MRM signals were detected. These enhanced product ion (EPI) spectra were searched against a mass spectral library for confirmation (Figure 3).

Figure 3: Quantitation of sulfamethoxazole and erythromycin in a river water sample and confirmation using enhanced product ion spectra and library search.
Analysis of BFRs
BFRs are used successfully in a variety of commercial products to reduce fire-related deaths, injuries, and property damage. However, there is concern because these emerging contaminants can be detected in environmental, human, and wildlife samples, even in locations far from where they were produced or used. LC–MS-MS can be used for the analysis of selected BFRs, including hexabromocyclododecane (HBCDD), which is used typically in polystyrene foam. LC–MS-MS allows the detection of HBCDD in different sample matrices and enables the study of the isomeric distribution of this environmental pollutant. The chromatograms in Figure 4 show an example of the detection of α-, β-, and γ-HBCDD in samples from an arctic seal and sediment. For this study, an API 2000 LC–MS-MS system (Applied Biosystems) was used with a C18 column and a gradient of water–acetonitrile–methanol and ammonium acetate buffer. The results show the difference in the isomeric pattern in biotic and abiotic samples because of preferential accumulation.
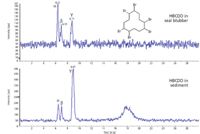
Figure 4: LC-MS-MS analysis of HBCDD, a brominated flame retardant in arctic seal and sediment samples, showing the different accumulation of different isomers.
Quantitation of Drinking Water DBPs
DBPs include chlorinated acetic acids, which are formed during drinking water production while treating water with chlorine and chloramine. Brominated acids can be formed when bromide ions are present in the source water. Additionally, water treatment with ozone can produce bromate ions. DBPs are analyzed in drinking water samples because of their potential human health risk, especially the risk of cancer.
These ionic environmental pollutants are easy to analyze in negative polarity with ESI on the API 3200 LC–MS-MS system (Applied Biosystems). But LC separation with sufficient retention is very difficult due to their high polarity. Ion chromatography (IC) coupled to MS-MS offers the ideal solution. The chromatogram in Figure 5 shows the analysis of a treated water sample with positive findings for monobromoacetic acid (MBAA), dichloroacetic acid (DCAA), bromochloro acetic acid (BCAA), and dibromoacetic acid (DBAA). A Dionex (Sunnyvale, California) ICS-2500 system with an IonPac AS20 column and a gradient of potassium hydroxide was used. In addition, a Dionex ASRS suppressor device was used to remove the buffer ions from the eluent before entering the ion source, which is necessary to avoid ion suppression during ionization and to maintain the robustness of the mass spectrometer.
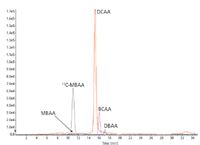
Figure 5: Treated water sample analyzed by IC-MS-MS for haloacetic acids.
Summary
LC–MS-MS allows the analysis of emerging environmental contaminants without time-consuming derivatization and with minimal sample cleanup. Highly polar organic compounds, such as PPCP, PFC, BFR, and DBP, can be quantified down to nanogram-per-liter levels, which is difficult or impossible using traditional techniques such as GC–MS or LC–UV.
The use of modern small-particle-size LC columns allows for increased throughput or improved chromatographic resolution. Triple-quadrupole mass spectrometers are used routinely for quantitation with selective and sensitive multiple reaction monitoring. Typically, a ratio of the quantifier and qualifier transition is used to confirm the presence of each identified compound. A recent trend is the screening for hundreds of contaminants in a single injection. Fast and powerful mass spectrometers are used for this type of analysis. Hybrid MS-MS, such as performed using QTRAP systems, offers unique analytical workflows. This includes the automatic acquisition of highly sensitive MS-MS spectra, which can be searched against existing LC–MS-MS libraries for confirmation.
Emerging environmental contaminants can be detected easily in various matrices at trace levels. We can expect that future developments will include more sensitive and robust hardware and testing methods and easier-to-use and more efficient data mining tools.
André Schreiber is with Applied Biosystems, Concord, Ontario, Canada.
References
(1) B. Vanderford et al., Anal. Chem. 75, 6265–6274 (2003).
(2) S. Richardson, Anal. Chem. 76, 3337–3364 (2004).
(3) S. Richardson and T. Ternes, Anal.Chem. 77, 3807–3830 (2005).
(4) S. Richardson, Anal. Chem. 79, 4295–4324 (2007).
(5) C. Borton et al., Application Note Applied Biosystems #114AP61-01 (2007).

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.











