The Use of Accurate Mass and MS-MS for the Analysis of PPCPs in Water
The Application Notebook
The United States Geological Survey (USGS) has found pharmaceuticals and personal-care products (PPCPs) containing known or suspected endocrine-disruptors in U.S. rivers. As such, it is important to use adequate techniques to help identify these compounds and possible metabolites.
The United States Geological Survey (USGS) has found pharmaceuticals and personal-care products (PPCPs) containing known or suspected endocrine-disruptors in U.S. rivers. As such, it is important to use adequate techniques to help identify these compounds and possible metabolites.
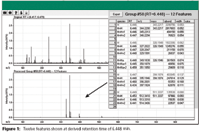
Figure 1
Using accurate mass of ions from time-of-flight (TOF) or quadrupole time-of-flight (QTOF), compound empirical formulas can be determined for purposes of identification. Although we have all used empirical formula, it has been pointed out to me that empirical formula is actually the reduced elemental composition (thus for the molecular formula C10 H20 O2, the empirical formula is C5 H10 O). Furthermore, the high degree of spectral resolution allows for selective identification among co-eluting compounds. Isotope ratios are an additional tool to help identify compounds with high carbon numbers as well as those that contain elements like chlorine and sulfur.
The Agilent QTOF has very accurate mass measurement at the MS-MS level, thus it is easier to determine the structures of the product ions, which correspond as substructures of the precursor ion and thereby reduce the number of possible structures pertaining to the derived molecular formulas from several to one.
Experimental
Prepared samples are provided by USGS in Denver, Colorado. An Agilent 6510 QTOF and Agilent 1100 Series LC with a ZORBAX RRHT Extent C18 column (2.1 mm × 50 mm, 1.8 μm) were used for the analysis. Please refer to Application Note 5989-7339 EN for method details.
Discussion
A typical full spectrum from a TOF (or QTOF) is very complex. As a lot of data are acquired by this type of instrument, it is important to have algorithms like Molecular Feature Extractor (MFE) that can identify usable ions out of the chemical background. These features are generated from spectra as a result of removing random background signal and finding clusters of isotopes that make sense. MFE derived 5,431 features from a water sample. A processed spectrum containing 12 features is shown in the lower half of Figure 1. The original complex spectrum is in the upper half of Figure 1. A subset of the list of features is shown at right. Compounds can be identified by searching the accurate masses of all these features against an exact-mass chemical database.
Once features are identified as compounds needing more structural information, or it is of interest to perform some quantitation, a targeted MS-MS analysis can be performed in which the ion mass of the feature is considered as the precursor ion and fragmented to form product ions. The accurate mass measurement of these product ions can be used to determine their chemical formula and possible structures. Because the QTOF also has a high degree of spectral resolution in MS and MS-MS modes, very narrow extracted ion chromatograms may be generated for each ion and then summed together for use as the quantitation signal.
Conclusion
The QTOF is a powerful instrument for identifying targeted and nontargeted compounds using accurate mass in single MS. The Molecular Feature Extractor (MFE) can determine usable features out of the chemical background. Compounds can be identified by searching the accurate masses of all these features against an exact-mass chemical database. Accurate mass targeted MS-MS of the extracted precursor ions provides both detailed structural information and quantitation.

Agilent Technologies Inc.
91 Blue Ravine Road, Folsom, CA 95630
Tel: +1 800-227-9770; Fax: +1 866-422-5571


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













