Quantitative Analysis of Pseudoephedrine Tablets by UHPLC-MS
The Application Notebook
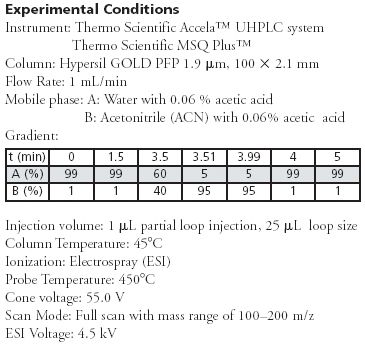
An effective UHPLC-MS method for high throughput separation, identification and quantitation of pseudoephedrine was developed on a Hypersil GOLD™ PFP 1.9 µm, 2.1 x 100 mm column.
An effective UHPLC-MS method for high throughput separation, identification and quantitation of pseudoephedrine was developed on a Hypersil GOLD™ PFP 1.9 µm, 2.1 x 100 mm column.
Pseudoephedrine and ephedrine are highly coveted by drug traffickers who use them to manufacture methamphetamine, for the illicit market (1). The separation and identification of pseudoephedrine from illicit drug mixtures, especially the mathaphetamine group compounds, will help to identify the sources and the manufacture pathway of the methamphetamine seized in the illicit market.
We report separation, identification, and quantitation of pseudoephedrine in a mixture of five illicit drugs/metabolites by ultra high performance liquid chromatography-mass spectrometry (UHPLC-MS).
Results and Discussion
Pseudoephedrine was identified as the major active ingredient for all the three brand name drugs by UHPLC-MS method (Figure 1). The peak retention time of 2.62 min for all three samples matched very well with the retention time of the pseudoephedrine standard at 2.60 min. The confirmation of pseudoephedrine at 2.6 min was further assured by the match of the MS spectra of the three samples with the pseudoephedrine standard.

Figure 1
An internal standard method was used for the quantitative determination of pseudoephedrine in its tablet form. The concentration of the assay samples determined (120.09 ppb) were in good agreement with the reported values (120 ppb).
Conclusions
A simple, fast, and reliable separation and identification method for five drugs (pseudoephedrine, ephedrine, amphetamine, methamphetamine and 3,4-MDMA) using UHPLC-MS is developed. The ppb (ng/mL) level sensitivity and accuracy by this method are more than sufficient to identify and quantify pseudoephedrine and/or other components in the seized illicit drug samples.
References
(1) Pseudoephedrine Notice, Office of Division Control, US Department of Justice, Drug Enforcement Administration.

Thermo Fisher Scientific Inc.
355 River Oaks Parkway, San Jose, CA 95134-1991
tel. +1 800-532-4752; fax +1 561-688-8731

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













