TSK-GEL ODS-140HTP, 2.3 µm: Ultra High Performance Reversed Phase Columns for High Throughput Analysis
The Application Notebook
Short analyses time and high resolution are in great demand from R&D and QC departments within the pharmaceutical industry. Sub-two micron ODS reversed phase columns have recently been introduced to meet these requirements, but these columns require an ultra-high pressure HPLC system to achieve optimum performance. TSK-GEL ODS-140HTP, 2.3mm columns from Tosoh Bioscience have been developed to offer a combination of short analyses time and high resolution separations that can be run at modest pressures, making these columns compatible with conventional HPLC instrumentation. The polylayer bonding chemistry of these columns results in highly efficient and physically stable columns when operated at high linear velocities. In addition, TSK-GEL ODS-140HTP, 2.3mm columns can be efficiently operated at pressures not exceeding 9000psi in UPLC® and other ultra-high pressure HPLC systems, as well as in traditional HPLC systems.
Short analyses time and high resolution are in great demand from R&D and QC departments within the pharmaceutical industry. Sub-two micron ODS reversed phase columns have recently been introduced to meet these requirements, but these columns require an ultra-high pressure HPLC system to achieve optimum performance. TSK-GEL ODS-140HTP, 2.3μm columns from Tosoh Bioscience have been developed to offer a combination of short analyses time and high resolution separations that can be run at modest pressures, making these columns compatible with conventional HPLC instrumentation. The polylayer bonding chemistry of these columns results in highly efficient and physically stable columns when operated at high linear velocities. In addition, TSK-GEL ODS-140HTP, 2.3μm columns can be efficiently operated at pressures not exceeding 9000psi in UPLC® and other ultra-high pressure HPLC systems, as well as in traditional HPLC systems.

Results
TSK-GEL ODS-140HTP, 2.3μm columns operate at lower pressure than competitive sub-2μm columns. This is illustrated in Figure 1. The pressure drop of a 5cm TSKgel ODS-140HTP, 2.3μm column at 25cm/min is more than 50% lower than that for smaller particle size competitive columns. Not surprisingly, the pressure drop over a 10cm TSKgel ODS-140HTP, 2.3μm column was still lower than any of the competitive 5cm columns.
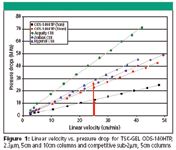
Figure 1
To demonstrate the physical stability of the TSK-GEL ODS-140HTP, 2.3μm columns, they were subjected to more than 1000 multi-step gradient cycles at high flow rate. Figure 2 shows that the number of theoretical plates remained basically unchanged for a TSKgel ODS-140HTP column during 1110 gradient cycles consisting of five min step gradients from 10% to 50% and from 50% to 100% methanol at 0.6mL/min. During each cycle, pressure fluctuated between 30 and 60MPa as a result of changes in mobile phase viscosity. Figure 3 shows injections of test solutes after the first step gradient cycle and after 1110 cycles. The results clearly demonstrate the durability of the TSK-GEL ODS-140HTP, 2.3 μm columns when operated at high flow rate and high pressure.
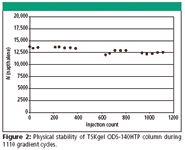
Figure 2

Figure 4 illustrates that high resolution of a peptide digest from beta-lactoglobulin can be obtained within minutes on a 2.1mm ID × 5cm TSKgel ODS-140HTP, 2.3μm column at a back pressure of about 35MPa (the highest pressure at about 15% acetonitrile in the gradient), i.e. still achievable in traditional HPLC systems. As expected, peak capacity improved when using a longer gradient time.
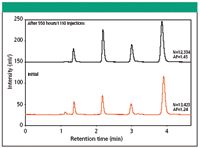
Figure 3
Conclusions
TSK-GEL ODS-140HTP, 2.3μm reversed phase HPLC columns from Tosoh Bioscience provide high resolution and short analyses times at moderate pressures, enabling high throughput separations. These columns were designed to be used with either UPLC® (up to 9000psi) or in conventional HPLC systems.
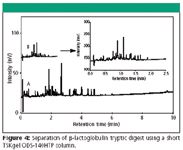
Figure 4
For use in high throughput applications, including drug discovery, pharmacokinetics and peptide digest separations, TSK-GEL ODS-140HTP, 2.3μm columns offer excellent resolution.

Tosoh Bioscience LLC
156 Keystone Drive, Montgomeryville, PA 18936
Tel. 215-283-5000 / 800-366-4875
Fax: 215-283-5035

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













