The Usage of Isopropyl Alcohol in Size-Exclusion Chromatography for Monoclonal Antibody Separation
The antibody drug market has continued to expand in recent years and will continue to do so for the foreseeable future. With this expansion, more antibodies will be designed and produced for targeting specific diseases. Size exclusion chromatography (SEC) is widely used to quantitate monomers, dimers, aggregates, and fragments in antibody analysis. Due to a high demand for better resolution and faster analysis time, more well-designed SEC columns have been introduced. These are 2 µm and sub-2 µm particle size SEC columns with the appropriate pore size for analyzing antibodies with optimized particle chemistry and column packing methods. Despite this improvement, nonspecific absorption of antibodies onto the column gel matrix poses a challenge, with some newly engineered antibodies possessing a high degree of hydrophobicity. The use of organic solvents such as isopropyl alcohol (IPA) or salts can decrease this interaction as reported by many scientists. However, the additives may alter the diffusion of these molecules, which results in retention time shift and poor peak resolution that did not occur in a typical aqueous buffer system, such as sodium phosphate buffer at neutral pH.
In this application note, a TSKgel(r) UP-SW3000, 2 µm SEC column was used for analyzing monoclonal antibodies (mAbs) with the addition of 15% IPA in sodium phosphate buffer, pH 6.7. As demonstrated, peak resolution and retention time shift were not impacted.

Experimental HPLC Conditions
Column: TSKgel UP-SW3000, 2 µm, 4.6 mm ID ? 30 cm
Instrument: UltiMate(r) 3000 UHPLC system run by
Chromeleon(r) (version 7.2)
Mobile phase: 15% IPA in 100 mmol/L KH2PO4 / Na2HPO4,
pH 6.7, 100 mmol/L Na2SO4, 0.05% NaN3
Flow rate: 0.30 mL/min
Detection: UV @ 280 nm
Temperature: 30 °C
Pressure: 22 MPa (maximum column pressure is 34 MPa)
Injection vol.: 5 µL, 4 mg/mL
Sample: USP mAb standard
Results and Discussion
The excellent reproducibility of injection to injection of the USP mAb standard onto the TSKgel UP-SW3000, 4.6 mm ID × 30 cm column, with a typical sodium phosphate buffer, pH 6.7, is shown in Figure 1. This figure is an overlay of 14 consecutive injections of the USP mAb standard sample at the flow rate of 0.3 mL/min. The retention times of monomer, dimer, aggregate, and fragment peaks are nearly unchanged. Peak width and peak shape are very consistent from injection to injection.
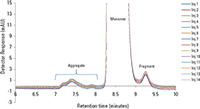
Table I consolidates the recorded calculated data from the monomer and dimer peaks of the 14 injections from Figure 1 with the %RSD of retention time and percent relative area below the allowance from the USP monograph guidance.
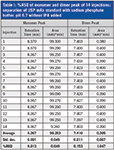
Figure 2 shows the overlay of 15 injections of the USP mAb standard sample onto the TSKgel UP-SW3000 column with the addition of 15% IPA. These injections are performed after the column is subjected to 15 injections of the USP mAb standard sample with sodium phosphate buffer, pH 6.7, without IPA. The baseline of the first injection (as shown in blue) indicates that the column takes only one to two injections to be stabilized. After that all subsequent injections are overlaid perfectly.
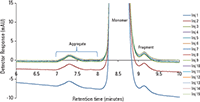
Table II lists the calculated data from the monomer and dimer peaks of the 15 injections from Figure 2 with the %RSD of retention time and percent relative area. As shown, the %RSD is below the allowance from the USP monograph guidance.
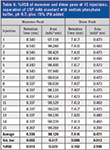
The retention times and peak areas from the injections without IPA added are very similar to the retention times and peak areas with IPA added (compare Table I to Table II).
At 0.3 mL/min, the pressure of the column is slightly higher when IPA is added to the mobile phase compared to when the column is operated without IPA. However, the pressure is only at 22 MPa with the IPA added. It is still far below the allowance of the maximum pressure of 34 MPa of the column's rating. With this low operating pressure, the TSKgel UP-SW3000 column can be operated with both HPLC and UHPLC systems. As the chromatograms indicate, all runs are completed within 15 min.
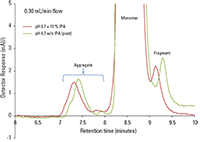
Figure 3 is an overlay of injections with and without IPA added to the mobile phase. The overlay indicates the similarities of peak retention times, peak width and peak height of dimer, monomer, aggregate, and fragment peaks between the two different conditions.
Conclusion
An appropriate percentage of organic solvent such as IPA does not alter the diffusion of mAb molecules using a TSKgel UP-SW3000 column. As demonstrated, this column can be successfully operated with the addition of 15% IPA. Data indicates that the column's particle chemistry and packing are optimized so that with the addition of an appropriate amount of selected organic solvents, there is no alteration of peak retention time or poor peak resolution.
Tosoh Bioscience and TSKgel are registered trademarks of Tosoh Corporation.
UltiMate and Chromeleon are registered trademarks of the Dionex Corporation.

Tosoh Bioscience LLC
3604 Horizon Drive, Suite 100, King of Prussia, PA 19406
tel. (484) 805-1219, fax (610) 272-3028
Website: www.tosohbioscience.com

A Guide to (U)HPLC Column Selection for Protein Analysis
April 16th 2025Analytical scientists are faced with the task of finding the right column from an almost unmanageable range of products. This paper focuses on columns that enable protein analysis under native conditions through size exclusion, hydrophobic interaction, and ion exchange chromatography. It will highlight the different column characteristics—pore size, particle size, base matrices, column dimensions, ligands—and which questions will help decide which columns to use.
The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.














