Under Pressure to Perform: Impact of UHPLC Technology on Pharmaceutical Research and Development
LCGC North America
How has UHPLC has affected pharmaceutical research? The answer depends on your point of view.
An informal survey of industrial chromatographers reveals some insights into the different ways that ultrahigh-pressure liquid chromatography (UHPLC) technology has had an impact on modern pharmaceutical research and development.
I recently spoke at the James L. Waters Symposium on Ultrahigh-Pressure Liquid Chromatography (UHPLC) at Pittcon 2019 in Philadelphia, Pennsylvania, where I had the honor of sharing the stage with Jim Jorgenson, Pete Carr, Ed Bouvier, and Bill Barber, key figures in the origin of UHPLC (1,2) (Figure 1). The James L. Waters symposium has become an important part of the annual Pittcon meeting and provides a window on the creation and evolution of a significant new analytical technology. Privately endowed by Jim Waters and now in its 30th year, this year's James L. Waters Symposium was organized and chaired by Steve Weber, who explained to me that my role was to describe the many ways in which UHPLC technology has transformed pharmaceutical research. While I had some personal opinions, the scope of the assignment led me to choose a survey approach, in which I discussed the matter with colleagues, then compiled the results into a short presentation. I received several requests to share this information, so I have reprised some of the content here for this article.

Figure 1: Speakers and organizers from the James A. Waters Symposium on ultrahigh-performance liquid chromatography (UHPLC) at Pittcon 2019, l to r, Stephen Weber, Peter Carr, James Jorgenson, Christopher Welch, Ed Bouvier, William Barber. Used with permission: Photo Copyright Notice: 2019 Roy Engelbrecht – royephoto.com
I recently retired from industry, and now, in the post-retirement phase of my career, I am leading the Indiana Consortium for Analytical Science and Engineering (ICASE), a joint venture between Purdue, Notre Dame, and Indiana University, focusing on research collaborations in measurement science and instrumentation. My work with ICASE often brings me into contact with industrial scientists, so I began the project by surveying friends, associates, and contacts in the pharmaceutical industry for testimonials and examples of how UHPLC technology has transformed research in their areas.
I soon learned that the "UHPLC advantage" means different things to different people. I spoke with chromatographers interested in analytical release testing, high throughput screening, drug metabolism, bioanalysis, drug discovery, formulation studies, and preparative separations. Researchers in drug discovery and early development often focused on speed advantages, while researchers from late-stage development, commercialization, and manufacturing often touted performance advantages relating to quality. There are many types of chromatographers in industry, some with formal training in the subject who know the equations and the theoretical underpinnings, and others who have learned on the job, developing a fine-tuned chromatographic sensibility derived from decades of practical experience. I set out to survey both extremes as well as those in the middle. Attribution is provided whenever possible, although a number of informants preferred to remain anonymous.
UHPLC Means Faster Separations
Naijun Wu, a well-known chromatographer from Celgene, provided an overview of UHPLC's impact on pharma, noting that in the drug discovery sector, fast "universal" sub-minute UHPLC methods for routine samples have now replaced the 5 to 10 min HPLC methods that were previously used. This increase in speed allows for faster decision making and overall shorter experimental cycle times.
Comparable speed advantages have profoundly changed the field of high throughput synthesis (where different catalysts and reaction conditions are rapidly screened) and in drug metabolism and pharmacokinetics studies (where thousands of plasma or urine samples are often analyzed). UHPLC speed advantages have also revolutionized in-process control (IPC) analysis, where 50 min end-of-reaction HPLC assays have been replaced by <5 min UHPLC methods. This faster turnaround allows for denser monitoring of reaction progress, and the ability to quickly intervene and adjust reaction conditions when necessary.
UHPLC has also allowed automated multicolumn screening for method development (such as six columns × three pH levels × two mobile phases) to evolve from an overnight or even multiday task in the era of HPLC to a job that can be accomplished in a single morning using UHPLC (3). The pace of research is greatly increased when method development can be performed within a single day, as opposed to waiting for several days.
The impact on the pharmaceutical commercialization and manufacturing sector has also been profound, with batch release (the task of ensuring quality of newly prepared batches of active pharmaceutical ingredients [APIs]) shrinking from two workday shifts in the HPLC era to a couple of hours with UHPLC. Naijun noted that this batch release constitutes more than a dozen chromatographic runs, for example, two blanks, eight system suitability samples, two reference standards, and three batch samples, analyzed in duplicate.
Several chromatographers from major pharmaceutical companies observed that it is important to place the UHPLC speed advantage in the proper context, noting that, in the days before the introduction of UHPLC, many separations in pharma were incompletely optimized, with 45 min purity assays and 10 min reaction screening methods being regarded by researchers as normal. In those days, it was not uncommon to see 10 min chromatographic runs used for monitoring single component dissolution assays. Simply put, optimizing the speed of HPLC separations was often not emphasized, perhaps owing to a less compelling need for speed and a historical emphasis on data quality, with researchers often doubling safety margins "just to be certain." When UHPLC arrived on the scene, there were some exaggerated claims of improvement in analysis speed, resulting from the comparison of nicely optimized UHPLC separations to essentially unoptimized HPLC separations.
Nevertheless, there is an actual UHPLC speed advantage compared to HPLC that can be profound. An example is shown in Figure 2, where both the HPLC and the UHPLC chromatograms are nicely optimized, resolution is comparable for the two methods, and substantial speed advantage for the UHPLC separation is observed.
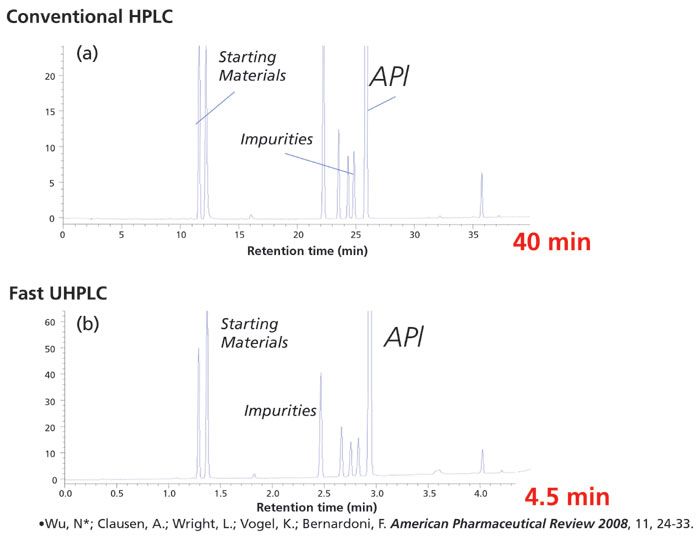
Figure 2: Fast UHPLC for reaction monitoring. Representative example of the speed advantage of (a) conventional HPLC analysis relative to (b) modern UHPLC analysis with a speed advantage of nine times. Conventional HPLC conditions: column 250 mm × 4.6 mm, 5-µm symmetry C18, 210 nm detection at 5 Hz, 10 µL injection, 40 °C, Agilent 1100, 1.0 mL/min 1450 psi, 60:40 to 5:95 1% phosphoric acid–acetonitrile in 40 min, 10 min post run. UHPLC conditions: column 100 mm × 2.1 mm, 1.7-µm Acquity BEH C18, 210 nm detection at 40 Hz, 2 µL injection, 40 °C, Waters Acquity system, 0.60 mL/min, 13,000 psi, 60:40 to 5:95 0.1% phosphoric acid–acetonitrile in 4.5 min, 2 min post run.
In this example, a ninefold increase in pressure (1450 psi for HPLC, 13,000 PSI for UHPLC) coincidentally affords a ninefold improvement in separation speed; however, several factors in addition to pressure and the nearly fivefold increase in linear velocity in the UHPLC method contribute to the observed speed advantage. In particular, the HPLC column is packed with 5 µm particles, whereas the UHPLC column is packed with 1.7 µm particles. Figure 3 illustrates the well-established improvements in chromatographic efficiency that are observed with columns packed with smaller particles (4). This increased efficiency generally leads to enhanced resolution, which can be traded for increased speed (5,6). In addition, the significantly lower extracolumn volume of current UHPLC instruments enables the use of smaller columns and faster gradients without a significant falloff in performance (7).
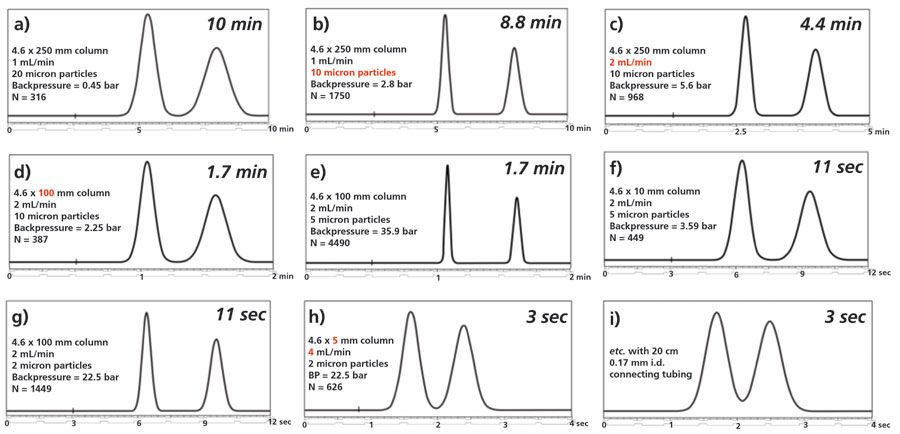
Figure 3: Impact of decreasing particle size on improving efficiency, resolution, and speed of chromatographic separations (4). Note chromatograms (a) through (i) with improvements in speed from 10 min to 30 s.
Irrespective of the various factors contributing to the UHPLC advantage, users have been quick to derive practical advantage from faster UHPLC techniques. An example of the tremendous gains in separation speed in recent years can be seen in the realm of chiral chromatography, which is primarily concerned with resolving the two peaks corresponding to the two enantiomers of a chiral substance (8). Figure 4 shows the historical evolution of the "world speed record" for resolution of the enantiomers of flurbiprofen, a representative chiral molecule. Other compounds show similar precipitous drops over time, reflecting dramatic improvements brought about by the introduction of new UHPLC and supercritical fluid chromatography (SFC) instrumentation, as well as introduction of new stationary phases containing smaller particles.
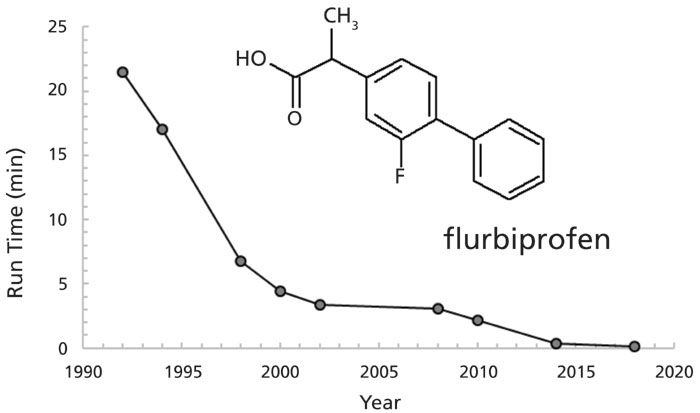
Figure 4: Evolution of "world speed record" for chromatographic resolution of the enantiomers of flurbiprofen over time. Advances in UHPLC and SFC instrumentation, new stationary phases, and smaller chromatographic particles have similar increases in speed for most chiral compounds. Data from ChirBase database.
Greener Separations Using UHPLC
Kelly Zhang and Pete Yehl from Genentech commented on the importance of speed, but also brought up another factor that was widely reported among respondents-the green chemistry solvent savings afforded by UHPLC. The green chemistry movement in pharma seeks to reduce the use of toxic materials and the generation of waste in the discovery, development, and manufacturing of pharmaceuticals (9). While the greatest impacts are often obtained in the manufacturing realm, green chemistry is very much an endeavor where "every little bit counts," thus chromatographers have been focusing for some time on the various ways that solvent use can be minimized during pharmaceutical analysis (10). Mike Hicks from Merck reported that UHPLC counts among the greatest contributions to green analytical chemistry, and that it has revolutionized modern pharmaceutical analysis. Christine Aurigemma from Pfizer noted that the advent of UHPLC, as well as next-generation SFC systems, means that solvent consumption and waste generation have been drastically reduced while separation speed has increased tremendously.
Faster Injections Needed to Compete With MS
Xiaoyi Gong from Merck cautioned that, while the UHPLC speed advantage has revolutionized many pharmaceutical workflows, UHPLC is still too slow for addressing many of the challenges of high throughput analysis. The pace of analysis of 96 well microplates was greatly increased by the development of fast analysis techniques such as MISER (multiple injections in single experimental run) (11), and multiplexed HPLC (12) and capillary electrophoresis (CE) (13) techniques. But the challenge of same day analysis of 1536 well microplates by UHPLC requires additional innovations. Conventional UHPLC analysis of 1536 samples currently takes more than 9 h, owing to limitations in the speed of injection via the robotic autosampler (14). A recent study reported a 2.4 h "plate time" for 1536 samples using MISER UHPLC–MS, although this approach relied on an eightfold mass spectrometry (MS) multiplexing strategy, in which groups of eight wells containing mass-differentiated products were combined prior to analysis (15). In contrast, direct MS measurement of 1536 well microplates can be completed in only 8 to 10 min using droplet microfluidics-MS (16), matrix-assisted laser desorption–ionization (MALDI) (17), or desorption electrospray ionization (DESI) (18) analysis. With the next generation of UHPLC separations sometimes taking less than a second (19), new ideas and approaches for sample injection will have to be developed to maintain the competitiveness of UHPLC for high throughput analysis.
Enabling the Investigation of New Modalities
In a phone conversation, Genentech's Pete Yehl pointed out that UHPLC has greatly enabled the exploration of the new therapeutic modalities that have been a significant focus for pharma over the past decade. Newly found resolving power provided by UHPLC was immediately put to use to study problems of ever-increasing complexity including small interfering RNA and other oligonucleotides (20), proteolysis-targeting chimeras (PROTACs), antibody–drug conjugates (ADC), and peptidic macrocycles. In addition, conventional small molecule drug candidates continue to increase in complexity, especially with regard to the presence of multiple stereocenters. Analysis and characterization of this next generation of pharmaceuticals is incredibly complex, taxing conventional HPLC and even the improved resolving power of UHPLC. Increasingly, multidimensional chromatographic separation approaches with orthogonal detection techniques are used to address these thorny problems (21,22).
Increasing Focus on Instrument Utilization
Several interviewees mentioned that the initial wave of UHPLC instrument introductions created an increased focus in pharma on instrument utilization rates, with instrument companies arguing that purchases of the more expensive UHPLC instruments would be justified by increased productivity, with a single UHPLC instrument doing the work of two conventional HPLC instruments. Irrespective of the merits of this argument or the actual UHPLC for HPLC replacement metric (several sources suggest that the actual ratio was more like 1:1), this focus on instrument productivity captured the attention of corporate procurement accountants and efficiency experts, who began to be increasingly involved in coordinating instrument lifecycle management.
Instrument utilization rates in certain areas of pharmaceutical discovery and development are often quite low, owing to the dedication of individual instruments to specific tasks, either on a project-by-project basis or on the basis of function-such as method development screening, walk-up "standard gradient" systems, and high throughput analysis systems. Consequently, the UHPLC revolution did little to either reduce instrument numbers or increase utilization. Going forward, improved software to better facilitate automated transitions between several discreet functions within a single instrument is an area of considerable interest among pharmaceutical researchers (3). Bill Farrell from Pfizer cites the series of solvent washes required to transition an instrument from reversed-phase mode to normal-phase mode and then back again as a potentially easily automated routine task that currently requires too much user oversight and guidance.
Larry Miller from Amgen reports that instrument utilization software that is now commonplace on newer HPLC and UHPLC instruments helps him track the number of injections for each instrument, which guides instrument maintenance and replacement scheduling, and also informs decisions on how best to place open-access instruments around the laboratories to best serve users. Larry claims that this data is also helpful when justifying the purchase of new instruments.
In the End, It's All About Performance
Tim Olah from Bristol Myers Squibb summed things up nicely by noting that for many researchers in pharma, it's all about performance. The significant impact of UHPLC on research operations has often been more about resolution and specificity than about speed (although, of course, these factors are linked). Tim and his colleagues have been able to develop and implement very sensitive and specific multiple component bioanalytical methods using UHPLC and MS detection that are capable of simultaneously quantifying therapeutic agents and definitive biomarkers in small biological samples, including tissue biopsies and dried blood spots (DBS) in a way that was simply not possible before the advent of modern UHPLC technology. They and other researchers are glad that UHPLC has become an integral technology that underpins research activities across the entire spectrum of pharmaceutical discovery, development, and manufacturing.
References
(1) J.E. MacNair, K.C. Lewis, and J.W. Jorgenson, Anal. Chem. 69, 983–989 (1997).
(2) J. Nawrocki, C. Dunlap, A. McCormick, and P.W. Carr, J. Chromatogr. A 1028, 1–30 (2004).
(3) F.T. Mattrey, A.A. Makarov, E.L. Regalado, F. Bernardoni. M. Figus, M.B. Hicks, J. Zheng, L. Wang, W. Schafer, V. Antonucci, S.E. Hamilton, K. Zawatzky, and C.J. Welch, TrAC 95, 36–46 (2017).
(4) C.J. Welch, ACS Cent. Sci. 3, 823–829 (2017).
(5) C.J. Welch and E.L Regalado, J. Sep. Sci. 37, 2552–2558 (2014).
(6) B. A. Rogers, Z. Wu, B. Wei, X. Zhang, X. Cao, O. Alabi, and M.J. Wirth, Anal. Chem. 87, 2520–2526 (2015).
(7) N. Wu, A C. Bradley, C. J. Welch, and L. Zhang, J. Sep. Sci. 35, 2018–2025 (2012).
(8) C.L. Barhate, L.A. Joyce, A.A. Makarov, K. Zawatzky, F. Bernardoni, W.A. Schafer, D.W. Armstrong, C.J. Welch, and E.L. Regalado, Chem. Commun. 53, 509–512 (2017).
(9) P.T. Anastas and M.M. Kirchoff, Acc. Chem. Res. 35, 686–694 (2002).
(10) C.J. Welch, N. Wu, M. Biba, R. Hartman, T. Brkovic, X. Gong, R. Helmy, W. Schafer, J. Cuff, and Z. Pirzada, TrAC 29, 667–680 (2010).
(11) C.J. Welch, X. Gong, W. Schafer, E.C. Pratt, T. Brkovic, Z. Pirzada, J.F. Cuff, and B. Kosjek, Tet. Asymm. 21, 1674–1681 (2010). P. Sajonz, W. Schafer, X. Gong, S. Shultz, T. Rosner, and C.J. Welch, J. Chromatogr. 1147, 149–154 (2007).
(12) Y. Zhang, X. Gong, H. Zhang, R.C. Larock, and E.S. Yeung, J. Comb. Chem. 2, 450–452 (2000).
(13) K. Zawatzky, C.L. Barhate, E.L. Regalado, B.F. Mann, N. Marshall, J.C. Moore, and C.J. Welch, J. Chromatogr. A 1499, 211–216 (2017).
(14) A. Buitrago Santanilla, E.L. Regalado, T. Pereira, M. Shevlin, K. Bateman, L-C Campeau, J. Schneeweis, S. Berritt, Z-C Shi, P. Nantermet, Y. Liu, R. Helmy, C.J. Welch, P. Vachal, I.W. Davies, T. Cernak, and S.D. Dreher, Science 347, 49–53 (2015).
(15) X. Diefenbach, I. Farasat, E. Guetschow, C.J. Welch, R. Kennedy, S. Sun, and J. Moore, ACS Omega 3, 1498–1508 (2018).
(16) S. Lin, S. Dikler, W.D. Blincoe, R.D. Ferguson, R.P. Sheridan, Z. Peng, D.V. Conway, K. Zawatzky, H. Wang, T. Cernak, I.W. Davies, D.A. DiRocco, H. Sheng, C.J. Welch, and S.D. Dreher, Science 361, 6236 (2018).
(17) M. Wleklinski, B.P. Loren, C.R. Ferreira, Z. Jaman, L. Avramova, T.J.P. Sobreira, D.H. Thompson, and R.G. Cooks, Chem Sci. 9, 1647–1653 (2018).
(18) M.F. Wahab, R.M. Wimalasinghe, Y. Wang, C.L. Barhate, D.C. Patel, and D.W. Armstrong, Anal. Chem. 88, 8821–8826 (2016).
(19) M. Biba, E. Jiang, B. Mao, D.Zewge, J.P. Foley, and C.J. Welch, J. Chromatogr. A 1304, 69–77 (2013).
(20) C.J. Venkatramani,S.R. Huang, M. Al-Sayah, I. Patela, and L. Wigman, J. Chromatogr. A 1521, 63–72 (2017).
(21) CL. Barhate, E.L. Regalado, J. Lee, N.D. Contrella, J. Jo, A.A. Makarov, D.W. Armstrong, and C.J. Welch, Anal. Chem. 89, 3545–3553 (2017).
Christopher J. Welch is with the Indiana Consortium for Analytical Science & Engineering, in Indianapolis, Indiana. Direct correspondence to: Chris.Welch@ICASE.center

A Novel LC–QTOF-MS DIA Method for Pesticide Quantification and Screening in Agricultural Waters
May 8th 2025Scientists from the University of Santiago de Compostela developed a liquid chromatography quadrupole time-of-flight mass spectrometry (LC–QTOF-MS) operated in data-independent acquisition (DIA) mode for pesticide quantification in agriculturally impacted waters.
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










