Mobile Phase Buffers in Liquid Chromatography (LC): Effect of Buffer Preparation Method on Retention Repeatability
LCGC North America
Many analysts have strongly held beliefs about buffer preparation methods, but these positions are not always supported by experimental evidence.
For liquid chromatography (LC) methods where the buffer pH and composition have an influence on retention, which buffer preparation method will provide the most repeatable results?
The measurement of pH, one of the most common of all analytical measurements, plays a major role in many chemical processes, affecting everything from the productivity of bioreactors in the biopharmaceutical industry, to the performance of separation methods in liquid chromatography (LC) and electrophoresis. Given that pH measurement is so common, I think we can be lulled into the perception that it is also simple, and that the pH reported by any benchtop pH meter can be accepted at face value under all circumstances. In my interactions working with people at a variety of experience levels over the years, I have often felt that people preparing buffers for use in LC are a little too trusting of the pH values reported by pH meters under ordinary circumstances. In preparation for this month's "LC Troubleshooting" column, my student Devin Makey and I performed some experiments to see if we could move in the direction of getting answers to questions around the topic of the "best" way to prepare buffers for use in LC. What follows here is a description of those experiments, and the data we observed. I believe that the results are interesting, and can support best practices for improving the reliability of LC methods. I know they are certainly affecting the way we operate in my laboratory, and I hope you will find them useful as well.
Dwight Stoll
Introduction
The Role of Eluent pH in LC
The pH of the eluent has a significant impact on retention and peak shape in several modes of LC separations. This is well understood in reversed-phase separations, where retention is strongly dependent on the solubility of the analyte in the organic–aqueous eluent. The pH of the eluent affects the charge state of various functional groups (COOH, NH2, and so forth), and the charge state of these functional groups has a major impact on the solubility of the analyte in water; this is the origin of the primary influence of pH on retention. For example, a simple weak organic acid like benzoic acid will be neutral (uncharged) in eluents buffered well below the pKa (~4.1), because the carboxylic acid functional group will be protonated. However, in eluents buffered well above the pKa, the carboxylic acid functional group will be deprotonated, and carry a negative charge. The strong interactions between the negatively charged carboxylate group and the highly dipolar water molecules result in a much higher water solubility of the benzoic acid in the high pH eluent, and thus lower reversed-phase retention under these conditions. This is exemplified by the experimental retention data shown in Figure 1 for benzoic acid on a C18 reversed-phase column, where the eluent was buffered at different pH levels.
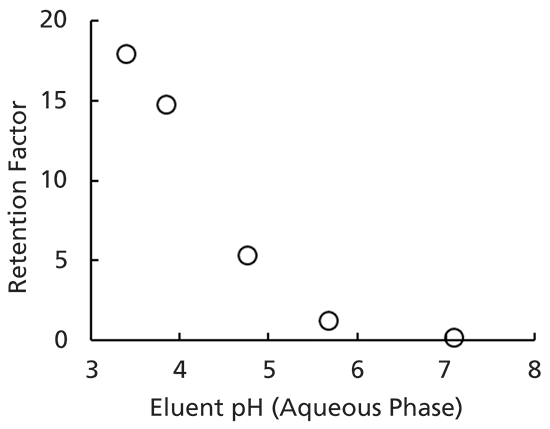
Figure 1: Effect of eluent pH on the retention of benzoic acid under reversed-phase conditions. Chromatographic conditions: column, SB-C18; eluent, 10:90 acetonitrile-phosphate buffer; temperature, 40 °C.
The same chemistry is relevant in hydrophilic interaction (HILIC) separations, though the dependence of retention on pH is often more complicated than it is in reversed-phase separations because of the more influential role of electrostatic interactions between the analyte and the stationary phase in HILIC. The influence of pH on other LC modes such as ion-exchange is even more evident because the magnitude of electrostatic interactions between the analyte and stationary phase is the dominant factor influencing retention. Thinking through these examples, it is clear that pH adjustment of buffered eluents is a topic with broad implications in LC.
Current Perspectives on Buffer Preparation
We also recognize that buffer preparation and pH adjustment is a pretty controversial topic in the LC community. This topic has been covered on multiple occasions in this column, focusing on aspects including buffer selection (1), preparation methods (2,3), and the idea of solution pH when an organic solvent is added to the mix (4). In a recent article of our own, we discussed the effects of different methods of buffer preparation on results from HILIC separations (5). In our preparation for this installment, we have found, through discussions with a variety of people, that they often have strongly held beliefs about what is right and wrong when it comes to buffer preparation, but also that these positions are not always clearly supported by experimental evidence. With this as a backdrop, we set out to make some of our own measurements with the hope that they would add to understanding in this area.
The most commonly used approach to buffer preparation for use in LC involves adding a salt of a buffering agent to water, then adding a small volume of relatively concentrated acid or base solution until a target pH is reached (as indicated by a pH electrode), and finally diluting to a specified volume. For example, suppose we are interested in a making 1 L of phosphate buffer at pH 6. Although there are many ways to prepare this buffer, a commonly used approach would be to first add sodium hydrogen phosphate (Na2HPO4) to about 900 mL of water. The pH of this initial solution will be about 9. Then, we could add phosphoric or hydrochloric acid to the solution slowly and watch the pH meter, stopping the addition of acid when the meter reads 6.00. We could then transfer the solution to a 1 L volumetric flask, and fill it to the mark with water. The focus of this article is really trying to answer the question, "If we make this buffer ten times, will we have added exactly the same amount of acid when we have stopped at pH 6.00, according to the pH meter?" If the answer is "yes," then all is well, and we should expect similar results from LC separations involving these ten buffers. We will show that more often than not the answer is "no," and that the extent of variation of the acid added from one buffer to the next is enough to cause measurable variability in retention in some cases. At this point you may be asking yourself, "How can the answer possibly be 'no'?" That in itself is a good question, and one that requires many more words than we can fit in this short article. We'll come back to this question at the end of our discussion, and suggest some reading material for those interested in really digging into this more. For now, on to the data.
Experiments, Results, and Discussion
Dependence of Retention on pH for Some Probe Molecules
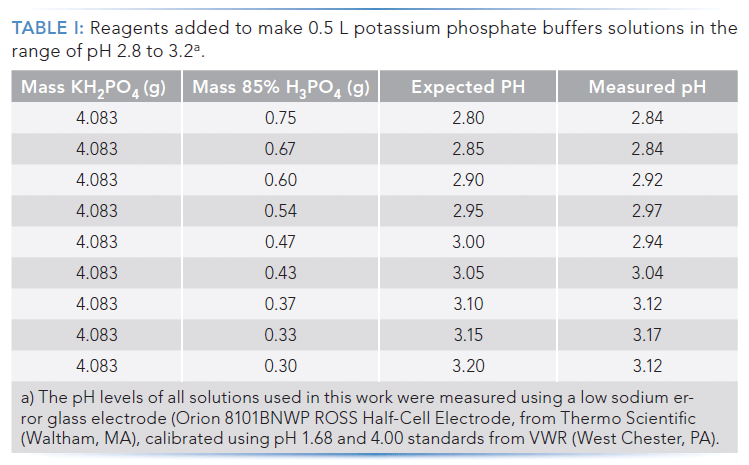
As a first step in this work, we set out to identify some simple probe molecules to use under reversed-phase conditions, and measure the dependence of their retentions on eluent pH. We chose one neutral molecule (ethylbenzene), one weak acid (butylbenzoic acid, pKa ~4.2), and two weak bases (4-hexylaniline, pKa ~4.8; and 4-aminobiphenyl, pKa ~4.3), and used uracil in our test mixture as a column dead time marker. We then prepared about 500 mL each of nine potassium phosphate buffers with expected aqueous pH values between 2.80 and 3.20, in increments of 0.05 units. The approach was to first add potassium phosphate (the same amount in each case, 30 millimoles), then add different amounts of phosphoric acid as needed to reach the target solution pH, and finally add enough water to reach a total mass of 500.0 g. These amounts are shown in Table I, and were calculated by solving the charge balance equation for this system for the number of moles of phosphoric acid that was required at each pH level. Activity coefficients were calculated using the extended Debye-Hückel equation (6).
Using each of these buffers as the aqueous component of the eluent, we measured the retention times of the four probe compounds on a C18 column. The resulting chromatograms for five of the buffers are shown in Figure 2, and a plot of the retention factors of the three ionizable probes relative to the retention factor of ethylbenzene is shown in Figure 3. At this point we make two observations. First, Figure 2 shows that the retention of ethylbenzene is nominally independent of pH, as expected, allowing us to normalize the retention of the other three probe compounds to the retention of ethylbenzene to minimize the effects of other variables such as temperature and organic to water ratio in the eluent on these measurements. On the other hand, the retention of the other three probes all exhibit some dependence of retention on pH, with the hexylaniline being the most sensitive of the three by far. Second, Figure 3 shows that the observed retention of each of the three ionizable probes is a smooth function of the calculated pH. Although the exact dependence of retention on pH is unknown for these conditions, we would at least expect it to be a smooth relationship.
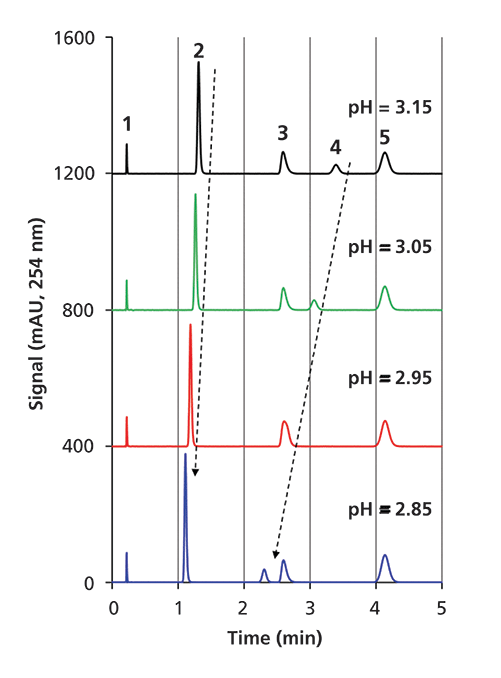
Figure 2: Effect of eluent pH on the retention of probe compounds (2) 4-aminobiphenyl, (3) n-butylbenzoic acid, (4) 4-hexylaniline, and (5) ethylbenzene. Uracil (1) was used as a dead time marker. Chromatographic conditions: column, StableBond C18 (50 mm × 4.6 mm i.d., 3.5-µm); flow rate, 2.0 mL/min.; eluent, 40:60 acetonitrile–buffer; temperature, 40 °C.
Comparison of pH Meter-Directed and Gravimetric Methods of Buffer Preparation
Now, let's return to our question above: "If we make this buffer ten times, will we have added exactly the same amount of acid when we have stopped at pH 6.00, according to the pH meter?" We prepared three replicates of a nominal pH 3 buffer as described in Table I by using two different methods:
A) pH meter-directed: In this case, we use the pH meter to decide when to stop adding phosphoric acid (for example, when the meter reads 3.00).
B) Gravimetric: In this case, we calculate the amounts each reagent ahead of time, and repeat that recipe each time, only measuring the pH of the solution when the buffer is complete.
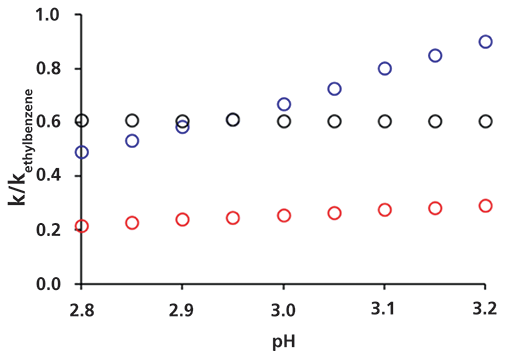
Figure 3: Retention of the three ionizable probes relative to the retention of ethylbenzene. Conditions are as in Figure 2. Key: O hexylaniline, O butylbenzoic acid, O aminobiphenyl.
The nominal procedures for the two methods, and the amounts of reagents added for the six buffers used to obtain the data shown in Figure 4, are shown in Table II.
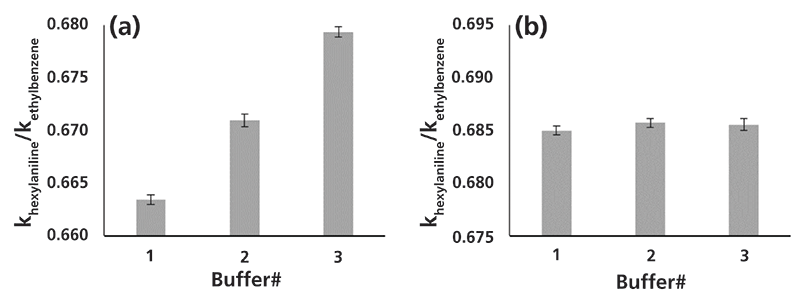
Figure 4: Relative retention of hexylaniline (normalized to ethylbenzene) for six buffers prepared by the same analyst; (a) for pH meter-directed approach, and (b) for gravimetric approach. Chromatographic conditions are as in Figure 2. Details of the buffer preparations are given in Table II. Error bars represent one standard deviation for ten replicate injections of the probe compound with a given buffer.
Figure 4 shows the mean relative retention of hexylaniline measured for six different buffers prepared by the same analyst, three by the pH meter-directed method (all using the same meter and electrode), and three by the gravimetric method. The results are quite clear. They show that the buffers prepared using the gravimetric method lead to much better repeatability of retention time in different buffers, relative to the repeatability observed for different buffers made using the pH meter-directed approach. These results are evidence that the answer to our question posed early in this article is "no". In other words, the pH values reported by the meter are not sufficiently repeatable to guide preparation of the buffer when buffers of highly repeatable composition are needed.
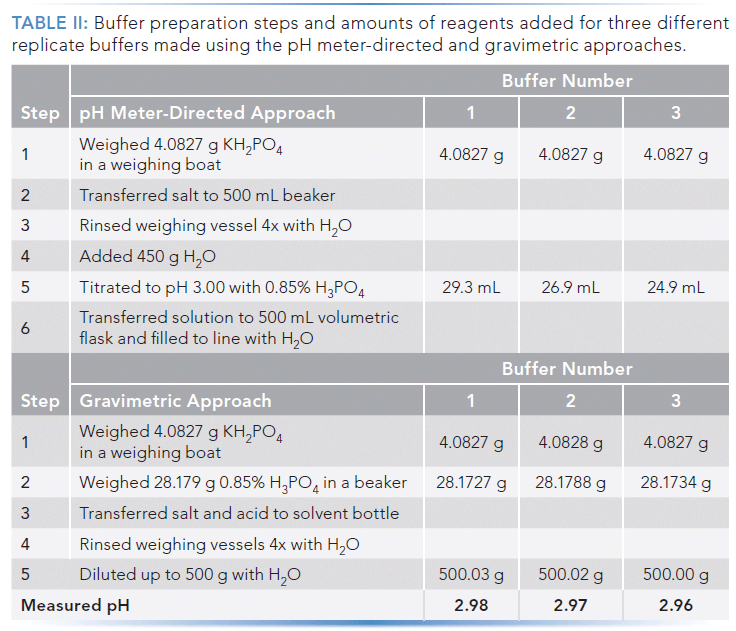
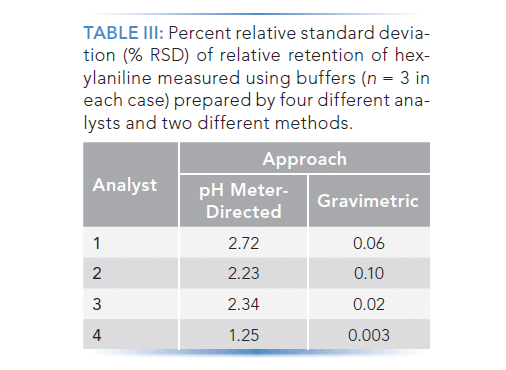
Having settled on the protocol shown in Table II for the gravimetric method, three other analysts from our laboratory each prepared three replicate buffers using the pH meter-directed approach, and three using the gravimetric approach. The results are shown in Table III, where we see that all four analysts were able to produce buffers that led to highly repeatable retention time using the gravimetric approach, whereas the buffers prepared using the pH meter-directed approach always led to much more variable retention times.
Closing Thoughts
Clearly not all work involving buffered solutions requires the level of repeatability in pH that we explored in this work. However, we believe these results show that, when working with analytes that have a significant retention dependence on pH of the eluent, the gravimetric approach to buffer preparation is worth considering seriously. Simply put, in most cases weighing reagents using a balance is a simpler operation than measuring pH using a glass electrode, and can be done with extraordinary precision compared to most other analytical methods. When the recipe for a particular buffer is known, and repeating the preparation of the buffer in a precise way is desirable, then the gravimetric approach is most precise. Readers interested in learning more about factors that influence the accuracy and precision of pH measurement at this level are referred to Bates's book on the topic (7). Finally, readers interested in tools for calculation of buffer recipes that can be used with gravimetric approach are referred to free web-based tools developed by Professor Rob Beynon (https://www.liverpool.ac.uk/pfg/Research/Tools/BuffferCalc/Buffer.html), and Professor Peter Carr and Aosheng Wang (http://zirchrom.com/Buffer.asp) It is important to recognize that the latter tool does not correct pH calculations to account for activity effects, which affect calculated pHs of solutions of high ionic strength and multiply charged buffer components (for example, phosphate at pH 7).
Acknowledgements
We would like to acknowledge the effort of Hayley Lhotka, Alex Florea, and Dr. Gabriel Leme and their willingness to participate in the experiments described here. We also thank Professor Peter Carr and Dr. William Tindall for their willingness to share their knowledge of this subject with us. DM was supported by a grant from the Camille and Henry Dreyfus Foundation.
References
(1) G.W. Tindall, LCGC North Am. 20, 1114–1118 (2002).
(2) J.W. Dolan, LCGC North Am. 33, 18–22. (2015).
(3) G.W. Tindall, LCGC North Am. 21, 28–32 (2003).
(4) G.W. Tindall, LCGC North Am. 20, 1028–1032 (2002).
(5) D.R. Stoll and C. Seidl, LCGC North Am. 36, 170–177 (2018).
(6) L.W. Potts, Quantitative Analysis: Theory and Practice (Harper & Row, New York, New York, 1987).
(7) R.G. Bates, Determination of pH: Theory and Practice (John Wiley and Sons, New York, New York, 2nd ed.,1973).
ABOUT THE CO-AUTHOR
Devin Makey

Devin Makey is an undergraduate student in his fourth year of study in chemistry at Gustavus Adolphus College in St. Peter, Minnesota.
ABOUT THE COLUMN EDITOR
Dwight R. Stoll

Dwight R. Stoll is the editor of "LC Troubleshooting." Stoll is a professor and co-chair of chemistry at Gustavus Adolphus College in St. Peter, Minnesota. His primary research focus is on the development of 2D-LC for both targeted and untargeted analyses. He has authored or coauthored more than 50 peer-reviewed publications and three book chapters in separation science and more than 100 conference presentations. He is also a member of LCGC's editorial advisory board. Direct correspondence to: LCGCedit@ubm.com

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








