ultrafleXtreme: Redefining MALDI Mass Spectrometry Performance
The new ultrafleXtremeâ„¢ exceeds all current expectations of MALDI-TOF/TOF technology: A proprietary kHz smartbeam-IIâ„¢ MALDI laser integrated with a novel FlashDetectorâ„¢ and re-engineerd electronics makes it the only MALDI-TOF/TOF on the market to provide kHz acquisition in MS and MS-MS modes. It generates a new level of data quality in applications such as LC-MALDI proteomics, high resolution tissue imaging based biomarker discovery or Top-Down Sequencing.
Ralf Schäfer, Bruker Daltonics Inc.
The new ultrafleXtreme™ exceeds all current expectations of MALDI-TOF/TOF technology: A proprietary kHz smartbeam-II™ MALDI laser integrated with a novel FlashDetector™ and re-engineerd electronics makes it the only MALDI-TOF/TOF on the market to provide kHz acquisition in MS and MS-MS modes. It generates a new level of data quality in applications such as LC-MALDI proteomics, high resolution tissue imaging based biomarker discovery or Top-Down Sequencing.

Figure 1
Advanced electronic engineering and a unique FlashDetector combined with 4 GHz digitization rate at full spectra length and "panoramic" PAN™ technology provide the highest resolving power >40,000 across the peptide mass range (Figure 1) and beyond (Figure 2). This leads directly to increased numbers of identified proteins in LC-MALDI applications at improved specificity.

Figure 2
The Ultimate Tissue Imaging Technology
The proprietary smartbeam-II laser with 1000 Hz repetition rate was specifically developed for the ultrafleXtreme MALDI-TOF/TOF. The software allows operation from 1 to 1000 Hz repetition frequency in MS as well as in MS-MS mode. Therefore, kHz data acquisition of high resolution tissue protein images and LC-MALDI analysis in Biomarker workflows are both perfectly sustained on the ultrafleXtreme. Compared to former instruments a full tissue analysis nowadays can be performed within the same data acquisition time (Figure 3).
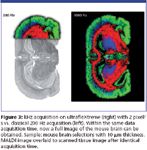
Figure 3
In addition, the optimized smartbeam-II laser delivers constant high output energy, regardless of the selected repetition frequency across long acquisition runs. This is indispensable for a series of MALDI imaging runs or LC-MALDI runs.
A computer-controlled variable laser focus diameter of 10–100 μm allows new level spatial resolution — essential for emerging applications like high resolution MALDI tissue imaging. Achieve incredible spatial resolution with no loss of protein signal intensity.
MALDI Perpetual™ Ion Source With Laser-Cleaning in Minutes
Entirely new design for the removal of sample and matrix debris. Its push-button, laser-cleaning concept is indispensible for kHz operation — and it works for all MALDI matrices. The self-cleaning ion optics is ready for the next run within a few min.
Market-Leading Technology
The combination of technologies implemented results in unmatched performance and productivity from the ultrafleXtreme MALDI-TOF/TOF:
- Patented 1 kHz smartbeam-II laser provides focus diameters down to 10 μm for high resolution imaging near the single cell level, at constant signal intensity even at small focus diameters.
- 1 kHz electronics engineered with precision high voltage control brings the laser performance to a true systems performance for all operational modes. From protein tissue imaging to LC-MALDI proteomic work, all fully enabled at 1–1000 Hz data acquisition rate for both TOF and TOF/TOF operation.
- PAN technology for high mass accuracy and resolution across a wide mass range, for precision proteomics and Top-Down protein sequencing in recombinant protein work.
- The new FlashDetector provides unmatched mass resolution and accuracy.
- The new 4 GHz digitizer, provides unmatched systems performance.
- The self-cleaning MALDI Perpetual ion source complements the suite of innovations ensuring robust operation.
Conclusion
The fusion of ultrafleXtreme's novel features shatters existing technological boundaries. The most flexible MALDI-TOF/TOF platform ever demonstrates its ability to be the reference class in MALDI-based Proteomics applications.
Literature
(1) Bruker Technical Note TN-15.
(2) A. Holle,A. Haase, M. Kayser & J. Höhndorf, Optimizing UV laser focus profiles for improved MALDI performance (2006) J. Mass Spectrom. 41: 705–716.
(3) Bruker Technical Note TN-32.
For research use only. Not for use in diagnostic procedures.

Bruker Daltonics Inc.
40 Manning Rd., Billerica, MA 01821
tel. (978)663-3660, fax (978)667-5993
Website: www.bdal.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)