Two-Dimensional Liquid Chromatography Does More Than Increase Peak Capacity
The Column
Two-dimensional liquid chromatography (2D-LC) is drawn out of the chromatographic toolbox if resolution for compounds of interest is insufficient. Recently, several studies have started to highlight 2D-LC as a tool of choice to streamline analytical workflows to increase automation making them less time-consuming. This article highlights two proven cases where 2D-LC does more than simply increase peak capacity.
Photo Credit: Olarn Meesang/Shutterstock.com

Two-dimensional liquid chromatography (2D-LC) is drawn out of the chromatographic toolbox if resolution for compounds of interest is insufficient. Recently, several studies have started to highlight 2D-LC as a tool of choice to streamline analytical workflows to increase automation making them less time-consuming. This article highlights two proven cases where 2D-LC does more than simply increase peak capacity.
Two-dimensional liquid chromatography (2D-LC) can be seen as the next logical step in liquid chromatography. To date, 2D-LC is predominantly considered when addressing unsolved chromatographic challenges; 2D-LC is considered the technique of choice when chromatographic resolution is insufficient, for example, when sample complexity is high and the number of analytes exceed peak capacity. In some cases (especially with comprehensive 2D-LC) this may result in a complex set of data requiring advanced results evaluation capabilities.
On the other hand, it is less well known that 2D-LC can also be used to streamline existing challenging analytical procedures, turning complex, manual interaction processes into automated, less timeâconsuming workflows. Decreased analysis time and substitution of manual sample preparation workflows, for example, when relying on manual sample desalting or fractionation-reinjection steps, have increasingly become areas of interest in the chromatographic community. One of the two chromatographic dimensions can be used to perform a sample preparation step that can be performed on-line, automatically and without time spent on sample transfer. This article highlights two case studies where significant reduction in analysis time was achieved by either substituting multiple one-dimensional (1D) separations or manual sample preparation by 2D-LC.
Case Study 1: Online Desalting Using 2D-LC for the MS Determination of Glucagon Analysis in Accordance with USP 39
Desalting often requires repetitive protocols, which are monotonous and time-consuming. Such protocols are errorâprone and lack good reproducibility, which is critical, especially in profiling or quantitation workflows. Moreover, they rely on disposable supplies and consumables, which are neither economical nor ecological when compared to on-line sample preparation workflows. A method was developed for the analysis of organic impurities in glucagon applying mass spectrometry (MS) detection (1). The first-dimension separation was in accordance with USP 39 but lacked MS compatibility, and was then followed by on-line desalting to enable MS detection in the second dimension of the 2D-LC analysis. The 1D assay and the analysis of the complex glucagon profile rendered it almost impossible to find an MSâcompatible eluent.
For an acceptable separation, the USP 39 LC–UV method used potassium phosphate in the mobile phase. This mobile phase was not compatible with MS detection but did provide excellent separation of glucagon and the four deamidated variants of glucagon. Deamidation is the loss of ammonia from asparagine or glutamine, respectively, and resulted in a +1 Da mass shift. Glucagon contains one asparagine and three glutamine side chains and deamidation is a common protein reaction during thermal stress. This glucagon analysis provided sufficient separation between the different analytes of interest, but only with ultraviolet (UV) detection. For more information on the analytes, MS detection and 2D-LC was required. An automated multiple heartâcut (MHC) approach was used, whereby all analytes were transferred into the second dimension. Automated desalting (diverting of the salt fraction off-line) and MS detection was then performed after the second dimension. Diverting the salt prior to the MS detection required a complete separation of the salt plug from the analyte of interest. This was possible by using a 2.1 × 12.5 mm column in the second dimension. Completed in accordance with USP 39, the retention time relative standard deviation (RSD) (n = 5) was 0.27% and peak area RSD (n = 5) was 0.11% compared to the suitability requirement of ≤2.0%.
In general, MHC 2D-LC can be used as an effective desalting solution to allow hyphenation of chromatographic methods applying MS-incompatible mobile phase to MS detection (2,3).
Case Study 2: 2D-LC–MS to Reduce Ion Suppression in the Determination of Cannabinoids in Blood Plasma
The analysis of cannabinoids in biological fluids, such as blood plasma or urine, is an important workflow for forensic toxicology laboratories. Detection of Δ-9 tetrahydrocannabinol (THC) and the corresponding metabolites in plasma are indicators of drug abuse. The main metabolites are carboxy-THC (THC-COOH) and hydroxy-THC (THC-OH). Both can be detected in biological matrices over a long period, therefore indicating a history of drug usage. THC itself is quickly metabolized and can be found only for a short time after THC consumption (4,5).
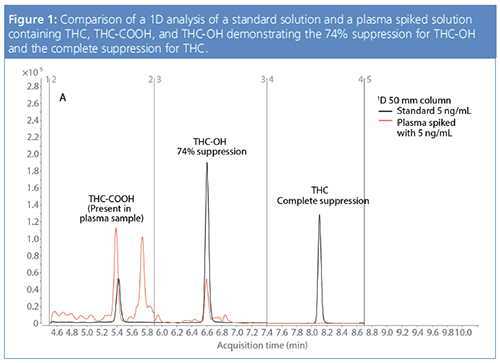
Analysis of these three substances in crude matrices, such as blood plasma, requires extensive sample preparation techniques such as protein precipitation, liquid–liquid extraction, solid-phase extraction (SPE), or a combination of the above. The primary reason for not applying direct LC–MS analysis for these types of samples is the large amount of matrix entering the ionization source. This impacts the ionization process, which can result in ion suppression. Moreover, the sample matrix can also lead to overall sensitivity reduction of the MS instrument by contaminating the ion source and the MS lens entrance over time. Therefore, matrix reduction or elimination is mandatory. On-line approaches to speedâup sample analysis time are therefore an attractive option. In another developed method, it was shown that MHC on the analytes of interest and analysis of the cut(s) in the second dimension with orthogonal separation principle significantly reduced the ion suppression effects for THC and the metabolites. In contrast, utilizing only a one-dimensional method resulted in an ion suppression for THC-OH of 74% and a complete suppression for the THC signal. This effect is illustrated in Figure 1. Without matrix removal, quantification was not possible. The only alternative approach to quantify THC in matrix following this workflow would be via the collection of fractions and re-injection on a second column–mobile phase combination with different selectivity. The complete THC peak was sampled in small fractions, and each of these fractions were analyzed automatically on a second-dimension column. In this case, a phenyl-hexyl stationary phase with formic acid and methanol as the mobile phase were selected. No ion suppression effects were observed using this 2D-LC approach. Good precision, linearity, and excellent recovery values were found for all three analytes (1).
Conclusions
In summary, 2D-LC will not only increase the peak capacity for complex samples, it can also reduce the overall analysis time and substitute manual steps by using the second dimension for desalting or removal of matrix. Moreover, the use of 2D-LC for desalting or sample cleanâup workflows is gaining more attention in the chromatographic community. As Petersson et al. state, they were able to optimize an existing protocol for the characterization of biopharmaceuticals in salt-based separation methods and shorten their workflow from days to hours by avoiding fraction collection and solvent exchange steps (6).
References
- G. Vanhoeacker et al., Two-Dimensional LC/MS/MS to Reduce Ion Suppression in the Determination of Cannabinoids in Blood Plasma (USA, Agilent Technologies, Inc., 2017).
- S. Krieger, 2D-LC as an Automated Desalting Tool for MSD Analysis (USA, Agilent Technologies, Inc., 2017).
- H. Luo et al., Journal of Pharmaceutical and Biomedical Analysis137(15), 139–145 (2017).
- M. Hädener, W. Weinmann, S. Schürch, and S. König, Analytical and Bioanalytical Chemistry408(7), 1953–1962 (2016).
- N.A. Desrosiers et al., J. Anal. Toxicol.39(4), 251–261 (2015).
- P. Petersson, K. Haselmann, and S. Buckenmaier, Journal of Chromatography A 1468(14), 95–101 (2016).
Andreas Borowiak is the Analytical HPLC Product Manager responsible for 2D-LC Solutions at Agilent Technologies located in Waldbronn, Germany. He holds a master’s degree in biochemistry and biophysics.
Jens Hühner is a former PhD student at the University of Tübingen (Tübingen, Germany) and Aalen University (Aalen, Germany). He has worked for several years on the coupling of chromatographic and electrophoretic separation techniques including mass spectrometric detection. Since February 2017, he has worked as a hardware and software product manager at Agilent Technologies in Waldbronn, Germany. In this role, he focuses on new and intuitive workflows for method scouting and capillary electrophoresis techniques.
E-mail:catherine.kaye@agilent.comWebsite:www.agilent.com

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










