TSK-GEL ODS-140HTP, 2.3 µm Columns for the Fast and Reliable Separation of Drugs Used in Cold and Sinus OTC Medicines
Since the USA Patriot Act* has been enacted, many pharmaceutical companies have reformulated their over the counter (OTC) drug products with phenylephrine (a nasal decongestant) as a substitute for pseudoephedrine. Phenylephrine comes as a tablet, a liquid, or a dissolving strip to take orally - all as a treatment for cold symptoms (1). Besides phenylephrine, most pharmaceutical formulations for common cold and sinus medications often contain multiple active ingredients to treat different types of symptoms in addition to numerous excipients. From an analytical perspective, the challenge is to develop chromatographic conditions that allow quantitative analysis of a variety of excipients that vary widely in hydrophobic properties.
Atis Chakrabarti, Tosoh Bioscience, LLC
Since the USA Patriot Act* has been enacted, many pharmaceutical companies have reformulated their over the counter (OTC) drug products with phenylephrine (a nasal decongestant) as a substitute for pseudoephedrine. Phenylephrine comes as a tablet, a liquid, or a dissolving strip to take orally — all as a treatment for cold symptoms (1). Besides phenylephrine, most pharmaceutical formulations for common cold and sinus medications often contain multiple active ingredients to treat different types of symptoms in addition to numerous excipients. From an analytical perspective, the challenge is to develop chromatographic conditions that allow quantitative analysis of a variety of excipients that vary widely in hydrophobic properties.
We used a 2.1 mm i.d. X 5 cm TSKgel ODS-140HTP, 2.3 µm reversed phase column to address the need to revalidate the test methods for new phenylephrine formulations with the overall goal to reduce retention time of the APIs (active pharmaceutical ingredients). Six different drug standards were selected covering a wide range of hydrophobicities, all of which are commonly used as APIs in many cold and sinus medicines. We report for the first time the separation of these six drug standards within a 4 min analysis time using a conventional HPLC system.

Figure 1
Experimental Conditions
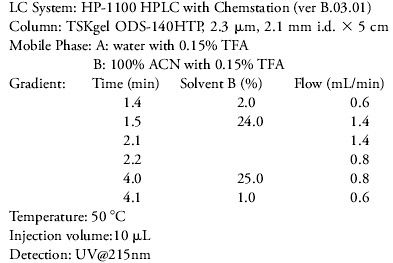
High purity Sigma brand chemicals were used for the preparation of stock solutions of phenylephrine, acetaminophen, doxylamine succinate, chlorpheniramine maleate, dextromethorphan hydrobromide, and diphenhydramine hydrochloride in 50% methanol (HPLC grade from Fisher). To avoid solvent mismatch at the time of injection, the working standards were prepared by diluting the stock standards in mobile phase A. The final concentration of each drug standard was 0.8 µg/mL in the drug cocktail. The standards were filtered through a 0.45 µm membrane prior to injection.
Results and Discussion
Six cold and sinus drug standards (Figure 1) containing phenylephrine (B), acetaminophen (C), doxylamine succinate (D), chlorpheniramine maleate (E), dextromethorphan HBr (F), and diphenhydramine HCl (G) could be separated as sharp peaks with good resolution within 3.8 min using a TSKgel ODS-140HTP column. The peak labeled (A) was identified as maleate originating from the drug standard chlorpheniramine maleate (E). The pressure drop over the 5 cm column was 12.5 MPa at the beginning and 16.3 MPa at the end of the gradient run. The retention time of diphenhydramine is considerably lower than that reported using an ACQUITY UPLC® system (2). The goal of most HPLC methods is to achieve baseline separations with a resolution of 1.5 or more for all key analytes - which was achieved in this study. The two drug substances diphenhydramine and dextromethorphan have very similar and strong hydrophobic properties with a tendency to co-elute or elute with considerable overlap. In this study they were separated with a resolution of 1.9.
The number of theoretical plates for drug standards D, E, F, and G were greater than 15,000. The reproducibility of the method was confirmed by calculating %RSD for retention time for 13 consecutive injections (data not shown).
Conclusions
In this study, it is clearly demonstrated that a TSKgel ODS-140HTP, 2.3 µm column can successfully be employed using a conventional HPLC system with operational pressure limit of 41.4 MPa to obtain a simple, fast, and reliable separation of a wide variety of drugs used in common cold and sinus OTC medicines with remarkable reduction of overall run time. Decreased run times help reduce the amount of solvent waste as well as analysis cost, which is particularly important during this time of acetonitrile shortage.
References
(1) http://www.nlm.nih.gov/medlineplus/druginfo/meds/a606008.html#brand-names.
(2) Mazzeo JR, LCGC Asia Pacific, 10(1), May 1, 2007.
*Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001.
Tosoh Bioscience, TSK-GEL, and TSKgel are registered trademarks of Tosoh Corporation.

Tosoh Bioscience LLC
156 Keystone Drive, Montgomeryville, PA 18936
tel. (215)283-5000, (800)366-4875, fax (215)283-5035
Website: www.tosohbioscience.com

Pick Your Poison. Isolation of Paclitaxel (Mar 2025)
March 7th 2025The diterpenoid, paclitaxel, which was identified as a potent chemotherapy agent for breast and ovarian cancer originates from the Pacific Yew tree. The isolation of paclitaxel from its major impurities is shown with the use of Hamilton’s PRP-1 (5 µm) HPLC column.