Tips & Tricks: GPC/SEC for Membrane Analysis
Membranes play an important role in living species as well as in technical processes, including in human care (for example, in kidney treatment). This instalment of Tips & Tricks discusses gel permeation chromatography/size-exclusion chromatography (GPC/SEC) for membrane analysis.
Photo Credit: Yagi Studio/Getty Images

Daniela Held and Peter Kilz, PSS Polymer Standards Service GmbH, Mainz, Germany.
Membranes play an important role in living species as well as in technical processes, including in human care (for example, in kidney treatment). This instalment of Tips & Tricks discusses gel permeation chromatography/size-exclusion chromatography (GPC/SEC) for membrane analysis.
Gel permeation chromatography/sizeâexclusion chromatography (GPC/SEC) is the standard technique to characterize soluble macromolecules in solution. It is an excellent method to separate molecules based on differences in size and can therefore be used to learn more about the filter capabilities of membranes. Membrane performance parameters, such as retention behaviour, pore size, and molar mass cutâoff, can be determined using standard chromatography equipment. An advantage of this approach is that membranes are tested under field conditions. This is especially important if swelling or nonâpermanent porosity has to be considered for the membrane quality.
Sample Preparation
This membrane characterization method is based on the filtration of a reference material of known size or molecular weight (1). If this material exhibits a broad molar mass or size distribution (large polydispersity index [PDI]), a single filtration experiment is sufficient to cover the complete pore size range of the membrane in the wet state. Using a material where the large PDI is a product of the synthesis process is preferred over mixing several samples with a narrow molar mass distribution because the broad molar mass distribution will be smooth and the materials are less expensive. The molar mass or size range of the reference material has to be selected so that some parts of it will be retained by the membrane, while others will pass through.
The reference material is then dissolved in the desired solvent to obtain a stock solution. Depending on if an organic or an aqueous solvent is used for the filtration process, polystyrenes, dextrans, or pullulanes can be used. The stock solution is filtered through the membrane applying proper filtration conditions (cross flow, back pressure). Molecules smaller than the membrane pores will be able to penetrate through the membrane into the filtrate, while larger molecules will remain on the feed side (retentate). The same stock solution can be used to test several membranes, which allows an easy quality control or comparison of different membrane types.Sample Analysis in GPC/SEC
Important membrane properties can be obtained by a specific comparison of the concentration profiles of the stock solution, filtrate, and retentate (optional, not required in some approaches). These collected samples have to be measured using standard GPC/SEC equipment, comprising of an isocratic pump, an injection system, and most preferably a refractive index detector (RI) to be able to detect pullulan or dextran. A conventional calibration of the system using standards of the same type as the reference material allows the molar masses to be determined.
Sieve curves can now be directly determined based on the obtained chromatograms of stock solution and filtrate (Figure 1). For this example the stock solution consisted of three dextran standards with different molar masses. If a dextran with a broad molar mass distribution had been used the chromatogram would not show maxima and minima but a steady distribution.
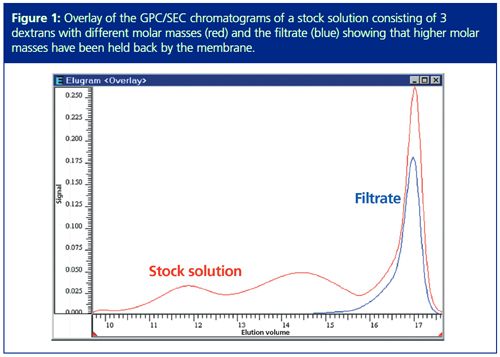
By overlaying the data of stock solution and filtrate it is obvious that the membrane held back the majority of the higher molar masses, which elute in GPC/SEC at lower elution volume. A part of the smaller molecules eluted (compare the peak at around 17 mL elution volume).
As in all cases, where chromatograms are compared, a reproducible elution is of utmost importance. Using an internal standard or flow marker can help to identify problems at an early stage. Correction using the flow marker increases the quality of data and results.
Results and Membrane Parameters
The next step is to quantify and to obtain important membrane parameters such as:
- sieve curve and retention efficiency
- average pore size and pore size distribution
- molar mass cut-off
- size selectivity
- pore accessibility
Sieve curves (S) are calculated from the stock solution (cS), the filtrate (cF), and (not required by all applications) the retentate (cR). An example equation can be:
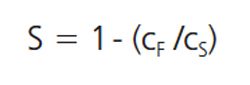
[1]
However, other approaches have also been described in the literature and can be applied to these data (2–6). Several membranes can be characterized in a sequence and their sieve curves can be easily compared.
Sieve curve molar masses can be assigned using the data from typical GPC/SEC calibration curves created, for example, using reference materials with narrow molar mass distribution (7). An additional parameter is the molar mass cutâoff of the membrane. This molar mass value defines the retention of a given am ple. Typically, 90% is used as the molar mass cut-off. However, additional retention parameters can be useful to answer specific questions or for in-depth comparison of different membranes. From the molar masses, the pore sizes can also be calculated using the Rg-M relationship with parameters taken from the literature (8,9).
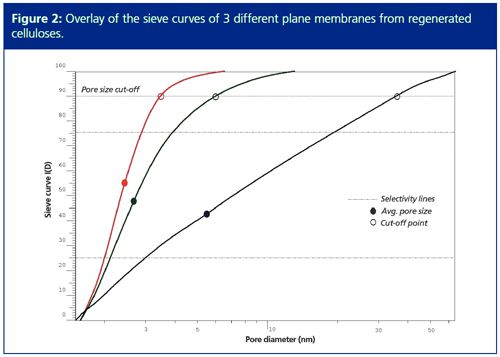
Figure 2 shows the graphical results for three different regenerated cellulose membranes, including the 90% cut-off results and the average pore size. All three membranes show very different filtration characteristics and even small property differences can be visualized with the overlaid data. The selectivity of a membrane, D, can also be calculated. Typically, this is determined at 25% and 75% retention. In the case of ideal selectivity, D = 1, the membrane would only allow a single size to pass through the pore. If no selectivity is achieved and the selectivity parameter is infinite, all sizes could migrate through the membrane.
Summary
- Standard GPC/SEC equipment can be used to characterize membranes. Stock solutions, filtrates, and retentates obtained by a filtration experiment are run as GPC/SEC samples.
- The use of a flow marker increases the data quality.
- Sieve curves can be calculated from the corresponding chromatograms.
- Molar masses can be taken for the calibration curve. Pore sizes can be calculated using the Rg-M relationship.
References
- H. Strathmann, in Separation and Purification Technology, N.N. Li and J.M Calo, Eds., (Marcel Dekker, New York, USA, 1992).
- P. Kilz, PMSE Preprints77, 56 (1997).
- M. Dai et al., Marine Chemistry62, 117 (1998).
- C. Causserand et al., Desalination250, 767 (2010).
- ASTM E1343-90, ASTM, West Conshohocken, United States (2001).
- French Standard NF X 45-103, AFNOR Association Française de Normalisation (1997).
- D. Held, The Column 4(6), 18–21 (2008).
- J.A.M. Smit et al., Macromolecules25, 3585 (1992).
- J. Brandrup et al., Eds., Polymer Handbook 4th edition (Wiley, New York, USA, 2003).
Daniela Held studied polymer chemistry in Mainz, Germany, and works in the PSS software and instrument department. She is also responsible for education and customer training.
Peter Kilz studied polymer chemistry in Mainz, Germany, and Liverpool, UK. He is one of the founders of PSS and head of the software and instrument department. He is also involved in customer support and training.
E-mail:Dheld@pss-polymer.com
Website: www.pss-polymer.com

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Quantifying Terpenes in Hydrodistilled Cannabis sativa Essential Oil with GC-MS
April 21st 2025A recent study conducted at the University of Georgia, (Athens, Georgia) presented a validated method for quantifying 18 terpenes in Cannabis sativa essential oil, extracted via hydrodistillation. The method, utilizing gas chromatography–mass spectrometry (GC–MS) with selected ion monitoring (SIM), includes using internal standards (n-tridecane and octadecane) for accurate analysis, with key validation parameters—such as specificity, accuracy, precision, and detection limits—thoroughly assessed. LCGC International spoke to Noelle Joy of the University of Georgia, corresponding author of this paper discussing the method, about its creation and benefits it offers the analytical community.











