Tips & Tricks GPC/SEC: Branching Analysis
The Column
Branching is one of the parameters chemists can adjust to produce polymer materials with optimized physical properties. Chromatography and advanced detection can help to characterize branched molecules. This instalment of Tips & Tricks explains more.
Photo Credit: Justin Dominic Alessandro Guisseppe Abella/ EyeEm/Getty Images

Branching is one of the parameters chemists can adjust to produce polymer materials with optimized physical properties. Chromatography and advanced detection can help to characterize branched molecules. This instalment of Tips & Tricks explains more.
An advantage of polymer materials is that their physical properties can be tailored for a specific application by adjusting many parameters. Besides composition, average molar mass, and the width of the molar mass distribution, another key parameter to control application properties is branching.
Branching requires at least a single branch point where three or more chains are connected. Branching can occur as an undesired side reaction during synthesis or it can be introduced deliberately to optimize the physical properties of the material. Different routes exist to synthesize defined structures, such as star-shaped or comb-shaped molecules.
The properties of branched polymers differ significantly from linear ones with respect to (melt) viscosity, glass transition temperature, the coefficient of bulk thermal expansion, solubility, and others. The property change depends on the parameters such as type of branching, length of the branches, and branching density.
The characterization of complex mixtures comprising not only a molar mass distribution but branching distribution as well, represents a real challenge. Depending on the type of branching there are various detection and separation options that provide deeper insight.
Gel permeation chromatography/size-exclusion chromatography (GPC/SEC) hyphenated with on-line viscometry1 (or less accurate multi-angle light scattering) can be used to characterize defined structures, such as star or comb-shaped polymers, or to investigate long chain branching. High temperature GPC (HT-GPC) with infrared (IR) detection can be used to investigate short-chain branching in polyolefins.2
For samples exhibiting broad molar mass distributions for branches and backbone and variations in branching density, the resolution of the size-based separation in GPC/SEC might not be sufficient to fully resolve the structures. Therefore alternative separation methods such as interaction chromatography (for example gradient polymer high performance liquid chromatography [HPLC] or temperature gradient interaction chromatography [TGIC]) or two-dimensional (2D) chromatography should be applied.3
GPC/SEC-Viscometry
An on-line viscometer is classified as a molar mass sensitive detector, however, the signal intensity is dependent on the viscosity rather than on molar mass. Viscometers provide direct access to the density of the molecules in solution. While the setup of such instrumentation, in most cases in combination with a light scattering detector, is very common, understanding the results and limitations requires more experience.
The Mark–Houwink plot is important for branching analysis; the logarithm of the intrinsic viscosity (obtained using on-line viscometry) is plotted versus the logarithm of the molar mass (obtained using universal calibration or light scattering detection). The slope of the Mark-Houwink plot (Mark-Houwink coefficient, αï¡) is dependent on the shape of the molecule in solution. If the intrinsic viscosity (solid sphere) has no molar mass dependence a slope of 0 is expected, however, the Mark–Houwink exponent of rigid rods is 2. Typical random coil polymers exhibit Mark-Houwink exponents in the range of 0.5 to 0.8, depending on solvent quality.
Branching analysis can be straightforward, assuming that the data can be compared to a linear chain of identical chemical structure and molar mass. Figure 1 shows Mark–Houwink plots for different polyethylene samples. A HT-GPC equipped with an on-line viscometer has been used to generate this plot. While the Mark–Houwink plot of the linear low-density polyethylene (LLDPE), which has only short chain branching, nearly superimposes with the linear sample, the low-density polyethylene (LDPE), which comprises long-chain branching, deviates significantly. At the same molecular weight the LDPE chains reveal a significantly lower intrinsic viscosity compared to the linear sample. This is a consequence of branches being present. The deviation increases with increasing branching density. By extrapolating the Mark–Houwink plots of the branched and linear polymer to a common intercept, it is possible to detect the molar mass at which branching first occurs. By taking the ratio of the intrinsic viscosity of the branched and linear polymer at the same molar mass, it is possible to determine the contraction factor, g', from which conclusions on the number of branches can be deduced.
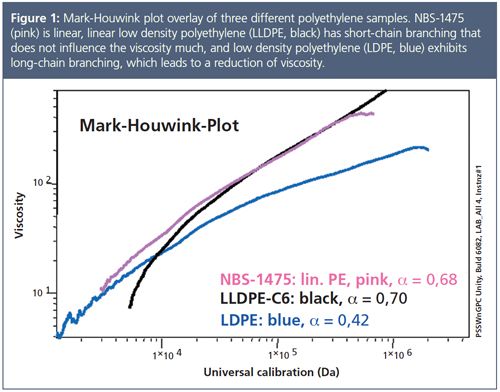
Figure 2 shows the results of GPC/SEC-viscometry of a poly(tert-butyl acrylate), PtBuA, star polymer. Star polymers are relatively simple branched polymers because they consist of several arms (linear chains) connected to a central core.
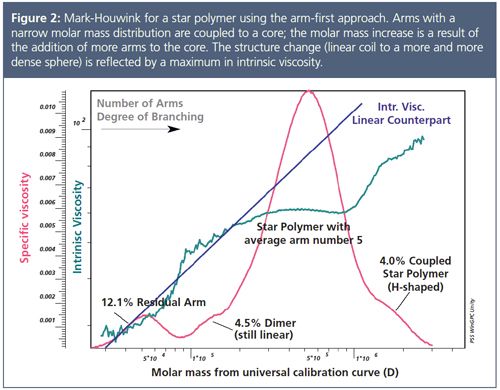
The star polymer was synthesized using the arm-first approach. PtBuA arms of narrow molar mass distribution have been coupled using a small amount of a bifunctional cross-linker to form the core. This means that the molar mass increase of the star was obtained by coupling an increasing number of arms of approximately the same length to the core. The Mark–Houwink plot reveals a maximum for the intrinsic viscosity, which has also been observed for dendrimers. Starting with a linear precursor, the coupling of two linear chains forms a still linear molecule, the dimer. Further reaction of precursor molecules with the core leads to three-arm and higher arm star polymers. Here the increase of the intrinsic viscosity with molar mass is counterbalanced by the decreasing intrinsic viscosity resulting from an increasing segment density with increasing number of arms for the branched structures.
This variation in molecular structure can be nicely monitored from the viscosity measured using an on-line viscometer. A very interesting observation is the sudden change of intrinsic viscosity occurring at approximately twice the molecular weight of the peak maximum. The drastic increase in viscosity is most probably a result of the formation of H-shaped molecules as coupling of two stars occurs via an arm.
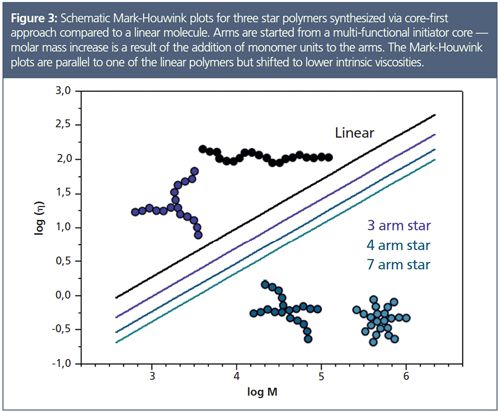
In contrast to the application above, star polymers can also be synthesized using the core first approach, for example, using a multifunctional initiator. In this case the observations and results would be different. There would be no structural change because the molar mass increase of the star would result from the growth of the arms. For such star polymers the Mark–Houwink plots are expected to be as shown in Figure 3. For each star polymer the Mark-Houwink plot will be shifted in parallel to lower viscosities at the same molar mass. Analysis would yield the same Mark-Houwink, αï¡, but a reduced Mark-Houwink, K (intercept). The shift to lower intrinsic viscosities increases with increasing number of arms.
Advanced Separation Techniques
One limitation of GPC/SEC is that it separates only based on the size of the molecule in solution. This has the following consequences for branched samples:
• Conventional calibration with reference materials will underestimate the molar mass of the branched samples. A solution here is the use of molar mass sensitive detectors, such as on-line viscometers or light scattering detectors. The viscometer can then be used for structure analysis and for molar mass determination based on universal calibration.
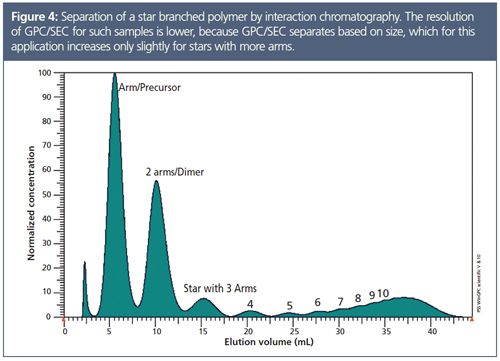
• In cases where the hydrodynamic volume increases only slightly with molar mass, such as arm-first star polymers, the resolution of size-based separations is limited. In this case interaction chromatography can be used as a complementary technique. Figure 4 shows the chromatogram of a gradient separation of an arm-first star polymer with a very high resolution even for stars with higher arm numbers.
• If samples exhibit broad molar mass distributions besides structural heterogeneity (for example, branched and linear chains present) the risk of co-elution increases. In that case branched molecules of higher molar mass with the same hydrodynamic size as linear chains of lower molar mass elute at the same retention volume. Consequently, the GPC/SEC fractions eluting from the column cannot be regarded to be monodisperse any longer.
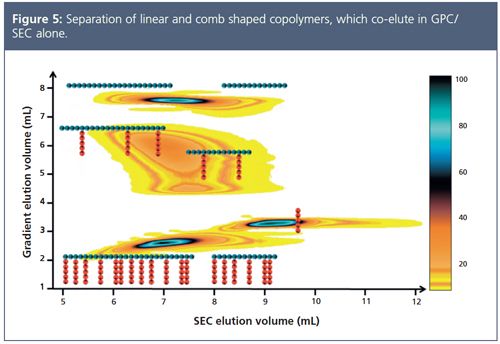
Comprehensive characterization of branched polymers might be possible when 2D separations are applied. Two-dimensional combines two independent separation techniques to generate contour plots, which can also be used to quantify the different species. Figure 5 shows an example where linear and comb shaped molecules of different composition have been successfully separated.
Summary
• Branching can occur as a side-reaction in polymerizations or can be introduced to tailor application properties.
• On-line viscometers provide access to the density of a molecule and can help to characterize branched molecules as they monitor structural changes with molar mass.
• Polyethylens exhibiting long-chain branches can be analyzed by on-line viscometry, while information on short-chain branching can be gained by Fourier-transform infrared spectroscopy (FT-IR) detection.
• Co-elution of branched and linear molecules can be a problem. In such cases advanced separation techniques or two-dimensional chromatography can be applied.
References
1. D. Held, The Column8(2), 12–16 (2012).
2. P. Montag, The Column12, 14–17 (2008).
3. J. Gerber and W. Radke, Polymer 46, 9224–9229 (2005) doi: 10.1016/j.polymer.2005.07.038.
Daniela Held studied polymer chemistry in Mainz, Germany. She currently works at the PSS software and instrument department and is responsible for education and customer training.
Peter Montag studied chemistry at the University of Duesseldorf, Germany, achieving his PhD at the Max Planck Institute for Coal Research. He is the head of the PSS contract analysis department and responsible for hyphenated techniques.
Wolfgang Radke studied polymer chemistry in Mainz, Germany, and Amherst, Massachusetts, USA. He is head of the PSS application development department and is also responsible for instrument evaluation and for customized training courses.

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).











