Is There Still Room for Innovation in Chiral Stationary Phases for Liquid Chromatography? The Fortunate Case of the Zwitterionic-Teicoplanin
This article reports on a recently developed zwitterionic-teicoplanin chiral stationary phase (CSP). As a result of the innovative chemistry used to immobilize the chiral selector, the CSP is characterized at neutral pH by the simultaneous presence of both a negative (carboxylate) and a positive (quaternary ammonium) charge. This feature has proved to be pivotal in efficiently and productively resolving very challenging problems. It is worth mentioning the simultaneous separation of chiral active pharmaceutical ingredients (APIs) from their counterions and the separation of neutral-nonpolar, neutral-polar, acidic, and basic N-Fmoc chiral amino-acids. Examples of highly-efficient ultrafast chiral separations achieved on this CSP, as a result of use of effective superficially- and porous-particles, are provided.
This article reports on a recently developed zwitterionic-teicoplanin chiral stationary phase (CSP). As a result of the innovative chemistry used to immobilize the chiral selector, the CSP is characterized at neutral pH by the simultaneous presence of both a negative (carboxylate) and a positive (quaternary ammonium) charge. This feature has proved to be pivotal in efficiently and productively resolving very challenging problems. It is worth mentioning the simultaneous separation of chiral active pharmaceutical ingredients (APIs) from their counterions and the separation of neutral-nonpolar, neutral-polar, acidic, and basic N-Fmoc chiral amino-acids. Examples of highly-efficient ultrafast chiral separations achieved on this CSP, as a result of use of effective superficially- and porous-particles, are provided.
The world of chiral stationary phases (CSPs) for liquid chromatography (LC) is very wide and continuously evolving. A universal CSP-that is, one able to resolve any kind of enantiomeric mixtures-does not exist. Literature is filled with examples of “novel” CSPs, whose applicability is, however, frequently very limited or even restricted to a single class of molecules. Very often, these CSPs have been obtained through modifications, either in the chemistry of the chiral selector or in the particle type employed as a support (fully-porous particles [FPPs] versus superficially-porous particles [SPPs]) (1–13). As a matter of fact, they are generally prepared by starting from already existing CSPs mostly belonging to the fundamental macroclasses of: antibiotics (8–11), proteins (5), Pirkle-type (1–4), and cellulose (6,7,12,13). In a large number of cases, advantages of novel CSPs are scarcely evident, over their predecessors.
Chiral separations represent a very challenging and complex research field. For a CSP to be successful, basic thermodynamic requirements-namely, the need of molecularly recognizing the enantiomers through the formation of diastereomeric transient complexes-must be balanced by important kinetic considerations, dictated by the (increasingly pressing) need of fast, highly-efficient separations (14,15). A proper combination of these two features can be very complicated to achieve. This explains why only a few of the novel CSPs become commercially available.
In chromatography, thermodynamics and kinetics can be singled-out through selectivity (enantioselectivity, in the case of separation of enantiomers) and efficiency, respectively. Enantioselectivity is chromatographically defined as the ratio between the retention factor of the more retained enantiomer over that of the less retained one. It describes the ability of a given CSP to separate (recognize) the enantiomers of a certain molecule. It is widely acknowledged that enantioselectivity directly depends on the specific loading of the chiral selector (expressed as µmol of chiral selector per square metre of silica). Generally, the larger the loading, the larger the enantioselectivity. The rationale for this is that the larger the surface coverage with selective sites, the smaller the contribution to retention (equal for both enantiomers) by nonselective ones (16,17). However, especially for chemically-bonded CSPs, that is, those where the chiral selector has been chemically anchored to the surface, it is not possible to predict the outcome of particle functionalization (in terms of loading) based on the particle properties, such as their specific surface area, nominal pore size, and even whether are they fully- or superficially-porous (14,15,18). On the other hand, chemically-bonded CSPs are the preferred choice compared to physically-coated ones, in virtue of longer stability and larger compatibility with different mobile phases (MPs). Enantioselectivity is not informative about the speed at which enantiorecognition occurs.
Separation efficiency is assessed as the height equivalent to a theoretical plate, or simply the plate height (H). H is an index expressing the rate of growth of peak variance along the separation path. Numerically, efficiency is expressed by the number of theoretical plates per metre (N/m), the higher the number the better the efficiency. Differently from achiral chromatography, in chiral chromatography, the kinetics of the adsorption-desorption can be very slow and its contribution to H relevant (14,15,18,19). Indeed, adsorption-desorption kinetics is often the dominant contributor to H in chiral chromatography. A slow adsorption-desorption process constitutes the real bottleneck to the realization of high-speed enantioseparations (18). Therefore, the use of either very small FPPs (sub-2-µm) or SPPs is not a warranty to achieve highly-efficient CSPs, per se.
The feasibility of a fast or very-fast chiral separation strongly depends on kinetic and thermodynamic properties of the enantiorecognition process of the analyte on the CSP. In addition, some experimental variables, firstly the MP composition, may dramatically affect the speed and efficiency of enantioseparations. It is worth mentioning cases where a change in the MP composition has induced the inversion in the elution order of enantiomers, with just as important consequences in terms of separation efficiency (20). Recently, many examples of fast or ultrafast chiral separations have been reported on macrocyclic-antibiotics (including teicoplanin) (21,22), cellulose-based (23), and Pirkle-type CSPs (especially Whelk-O 1) (1–3,18).
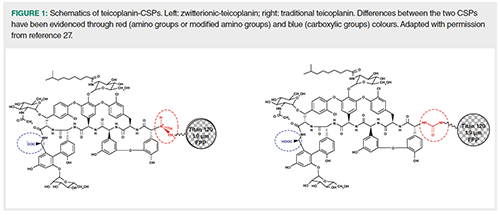
This article focuses on a new teicoplanin CSP prepared by means of an innovative bonding strategy (22,25,26) that has allowed to obtain a zwitterionic-version of the well-known CSP designed by Armstrong and coworkers more than twenty years ago (16,24). The zwitterionic CSP was designed with the aim of eliminating, or at least reducing, some limitations of traditional teicoplanin, such as the phenomenon of Donnan’s exclusion of anions from the CSP (27,28). At neutral pH, the novel CSP is characterized by the simultaneous presence of both a negative and a positive charge (Figure 1). The first comes from the deprotonation of the carboxylic moiety as it happens on the traditional version of the CSP (evidenced in blue in Figure 1). The positive charge is instead due to the protonation of the nitrogen atom of the secondary amine that replaces the neutral ureido-group of the traditional teicoplanin (represented in red in Figure 1). This difference has profoundly impacted the behaviour of the zwitterionic-teicoplanin CSP. As will be demonstrated by study cases reported below, indeed, the zwitterionic-teicoplanin has allowed to significantly expand the applicability of teicoplanin-based CSPs to very challenging and important separations, which could not be faced by the traditional teicoplanin.

Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
New Study Investigates Optimizing Extra-Column Band Broadening in Micro-flow Capillary LC
March 12th 2025Shimadzu Corporation and Vrije Universiteit Brussel researchers recently investigated how extra-column band broadening (ECBB) can be optimized in micro-flow capillary liquid chromatography.





