The Future of Biomolecule Analysis Using HPLC/UHPLC
The evolving future of biomolecule analysis using high performance liquid chromatography/ultrahigh-performance liquid chromatography (HPLC/UHPLC) is discussed in this article.
Biotherapeutics were historically only biotechnologically-produced pharmaceuticals. These include recombinant proteins, such as enzymes, hormone peptides, growth factors, and monoclonal antibodies (mAbs). As a result of the advances in bioengineering, molecules are currently designed to be safer and/or more efficient (1). The following modifications have been introduced to achieve optimized molecules:
- Reduction in size for better tissue penetration (for example, single-chain variable fragments [scFv] or nanobody instead of full antibody);
- Combination of different proteins to achieve multiple functionalities, for example, bispecific antibodies, fusion-proteins;
- Glycoengineering to alter pharmacokinetic characteristics or biological activities, for example, afucosylated mAbs;
- PEGylation to increase serum half-life, for example, of antibody fragments.
背景素材:近未来的な粒子やグリッターのパーティクル抽象背景Generative AI | Image Credit: © おでんじん - stock.adobe.com

New Molecules
In addition to optimizing protein-based therapeutics, novel modalities are entering the pharmaceutical market—though not all of them are entirely biotechnologically produced. Amongst them are nucleic-acid-based therapeutics, such as antisense oligonucleotides, small interfering ribonucleic acids (siRNAs) (chemically synthesized), or messenger RNA (mRNA) (in vitro transcribed). Some of them are administered in delivery particles such as lipid nanoparticles (LNPs). These are much larger (50–200 nm in diameter) compared to conventional biotherapeutics (mAb 5 nm diameter). This large size is a characteristic LNPs share with gene delivery particles. The major vector used is adeno-associated viruses (AAVs) of 20–25 nm, which are composed of a genetic cargo in a shell comprised of capsid proteins. Other particles used as therapeutics rely solely on a virus-like shell to induce an immune response and are used as a vaccine. These molecules use virus proteins to build a virus-like particle (VLP).
Challenging Conventional Analysis of Biotherapeutics
A set of chromatographic modes has been established for routine and quality control (QC) analysis of classical biopharmaceuticals. Some of them are particularly gentle to proteins because they leave them in their native state (secondary/tertiary/quarternary structure), while others lead to protein denaturation.
Native separation methods include:
- Size-exclusion chromatography (SEC) for aggregation/fragmentation analysis;
- Ion-exchange chromatography (IEC) for charge variant analysis;
- Hydrophobic interaction chromatography (HIC) to determine overall hydrophobicity or oxidations, deamidations.
Denaturing separation methods include:
- Hydrophilic interaction liquid chromatography (HILIC) for glycan analysis (performed on released glycans, not on intact proteins);
- Reversed-phase chromatography for peptide mapping and primary structure analysis (performed on enzymatically digested proteins).
These conventional methods are challenged by current trends, which include:
- Higher complexity molecules and impurities;
- Similarity between product and product-related impurities;
- Molecules composed of two or more different biological molecule classes;
- Increased molecule size;
- Demand for higher efficiency (savings on solvents, time, and costs).
The Future of Biomolecule Analyses
These challenges are tackled by various means, which are outlined below.
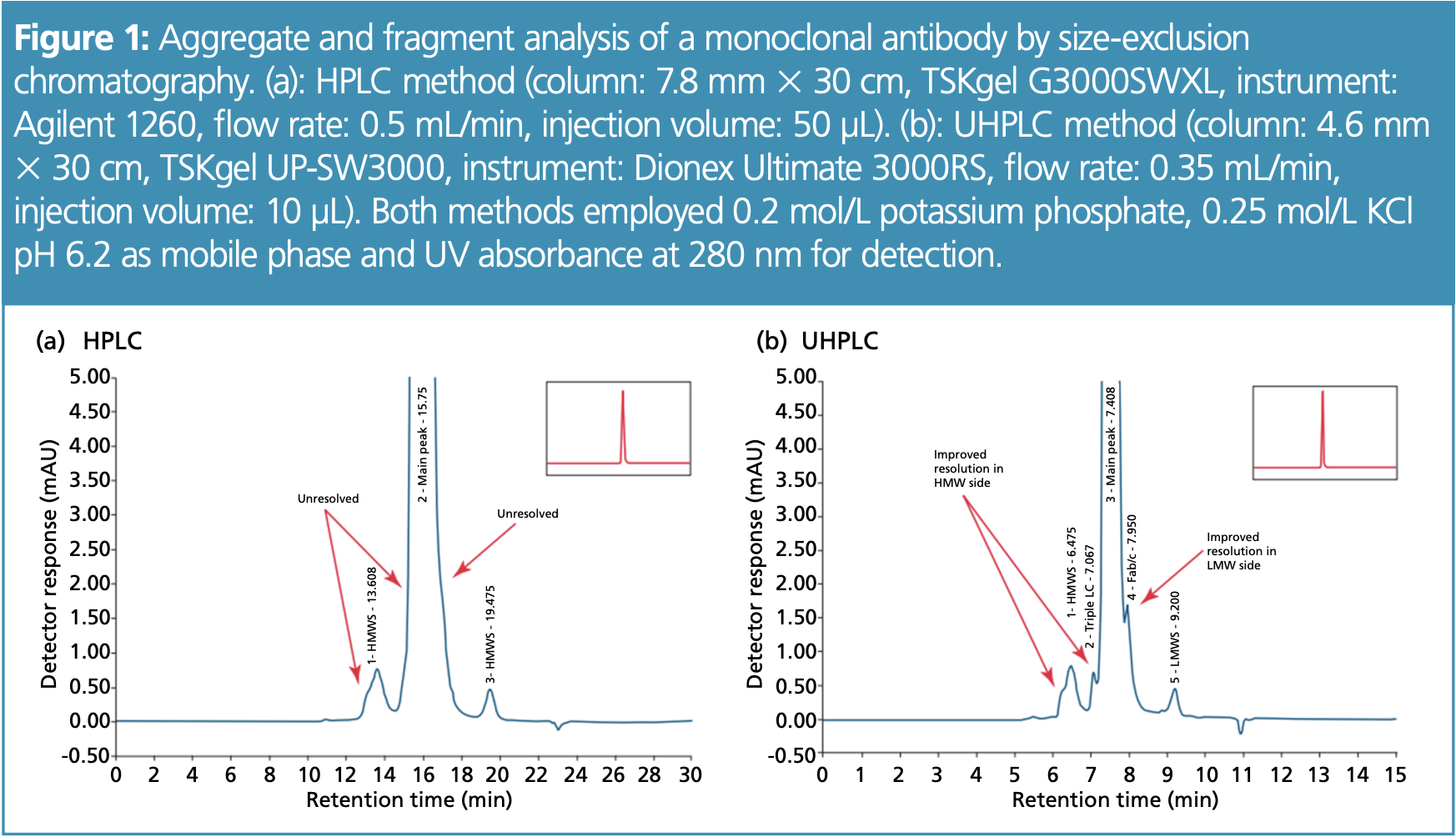
Higher Complexity—Better Resolution with Ultrahigh-Performance Liquid Chromatography (UHPLC): It is now commonplace to analyze more complex samples with UHPLC instead of high performance liquid chromatography (HPLC) to increase resolution and save analysis time and solvents. In this way, impurities that are similar to the desired molecule or are only present in small concentrations can also be separated. Figure 1 shows the separation of an antibody sample by SEC to determine the proportion of aggregates (high-molecular-weight impurities) and fragments (low-molecular-weight impurities). Peaks and shoulders only became visible using the UHPLC method and could not be determined with the HPLC method.
Higher Complexity—More Information Using Two-Dimensional (2D)–LC and New Separation Modes: The more that the impurities and the desired product resemble each other, the more difficult it becomes to separate them—independent of the chromatographic mode applied. To overcome that limitation, two separation modes can be combined to take advantage of orthogonal separation modes and increase selectivity (2). Popular combinations for biotherapeutics are the use of a native separation method for the first dimension, such as HIC, IEC, or SEC, and reversed-phase chromatography as a second dimension (3). Current 2D–LC developments are focused on improving the robustness of 2D–LC, facilitating method development and overcoming limitations such as buffer incompatibilities between two combined methods or undersampling (4,5).
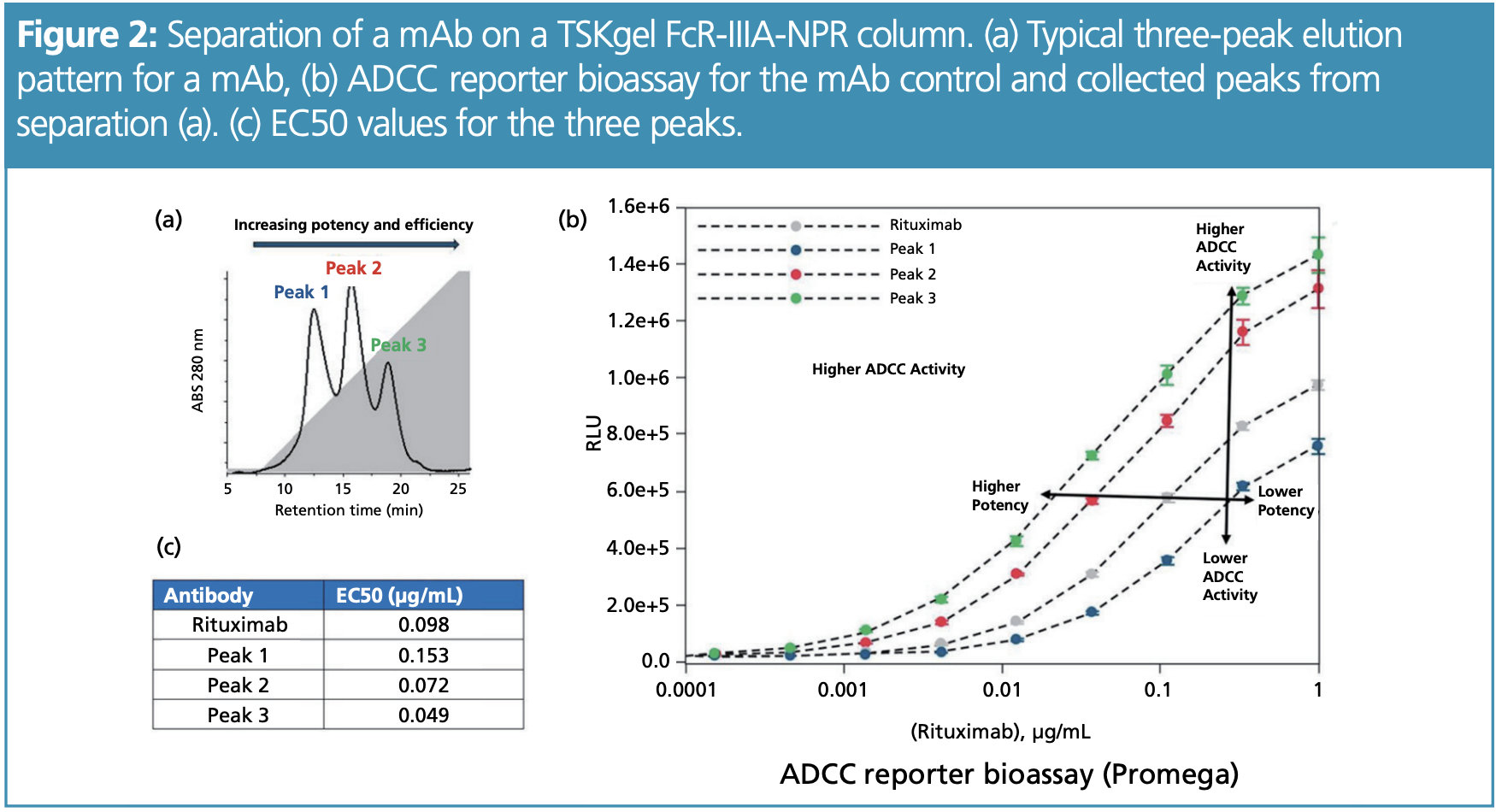
Instead of combining two chromatographic methods, the extension of the chromatographic toolbox provides additional information on the sample composition. One possibility is to use affinity chromatography, which mimics biological interaction processes. By using functionally relevant proteins as a ligand on HPLC or UHPLC columns, biotherapeutics can be separated for a particular function. An example is the use of stationary phases with Fc receptor ligands. These receptors are expressed by immune cells to convert the presence of antibodies into immunological responses; using them as a stationary phase demonstrates the ability of mAbs to elicit immune responses. Figure 2(a) shows the HPLC separation of a mAb sample separated on an affinity column with Fc gamma IIIA receptor ligand. An antibody sample was separated into subsets with different affinities for the receptor, which correlated with the ability to induce antibody-dependent cellular cytotoxicity (ADCC)—confirmed by a cell-based reporter assay (Figure 2[b]).
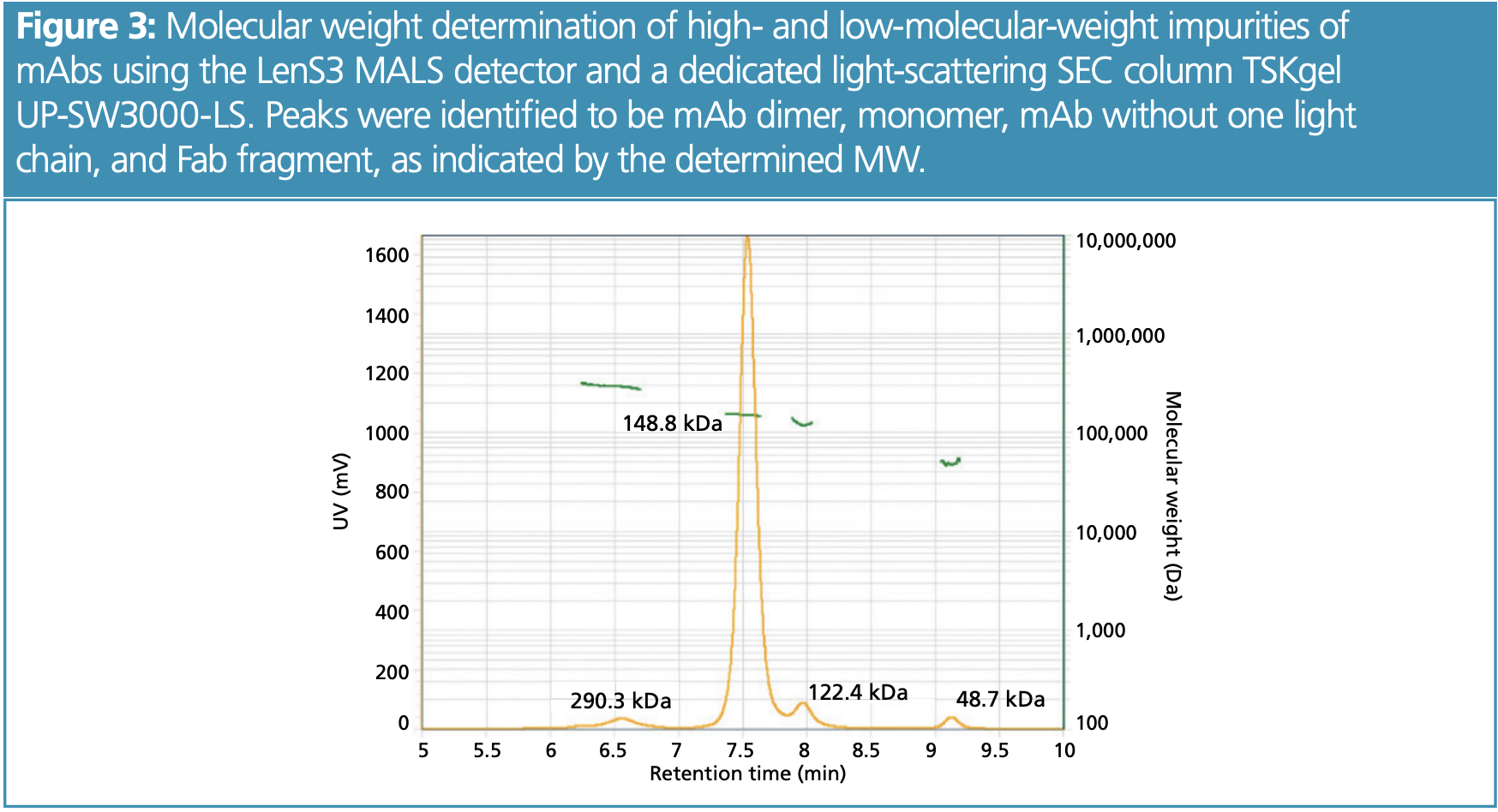
Higher Complexity—More Information Due to Advanced Detection Methods: Another approach to investigate new biotherapeutics— from multi-specifics to delivery particles—is to combine chromatographic separations with detection methods that deliver more information than UV detectors, such as multi-angle light scattering (MALS) and mass spectrometry (MS). Both detectors can be connected in-line with the chromatography instrument and are capable of determining molecular weights. MALS is easier to combine, as it does not require method adjustment and can follow any isocratic separation. It measures the scattering properties (intensity, intensity at different scattering angles) to determine the molecular weight and the gyration radius (size) of the separated sample. The separated molecules can therefore be characterized more accurately and chromatography deficiencies can be compensated for. This applies, for example, to SEC separations in which undesired interactions take place, when peaks elute closely to each other, or the molecule size relates differently to the molecular weight (MW) than a calibration standard. In those cases, the elution time does not correlate with the MW of a peak. MALS determines the MW independent from elution time and facilitates peak identification (Figure 3). While MALS is capable of determining the MW with an accuracy of 1–5%, MS is capable of more accurately determining the MWs of sample components down to 0.001 Da. This higher accuracy can be used to determine either different post-translational modifications, such as the glycan pattern, or to determine a protein’s primary structure (amino acid sequence) when separating its peptide digest. However, the separation method often has to be adapted because the detection takes place in the gas phase and volatile buffers have to be used.
While both of the detection methods (MALS and MS) are employed in research and development, they have not found their way into routine QC analysis yet. However, efforts are being made to develop more reliable and robust methods to use MS or MALS routinely and to benefit from the additional information they provide. This is supported by intuitive software to facilitate instrument use, or dedicated HPLC/UHPLC columns to use with advanced detection methods.
Mix of Molecules (Lipids, Nucleic Acids, Proteins, PEG)—Different Detectors Clarify Composition
As more and more drugs combine different molecule classes such as mRNA and lipid nanoparticles or PEG and protein, it is necessary to analyze how much of which molecule type contributes to the mixed targets. To this end, it is exploited that different molecules respond differently in detection methods such as UV280, UV260, or refractive index (RI). If it is known how a single (non-complexed or non-conjugated) molecule responds, the contribution of two molecules can be derived from combining two different detectors. For instance, this is used to determine the PEGylation state of proteins or the amount of cargo packed within a delivery particle.
Large Molecules—Larger Pore Sizes and Non-Porous Materials
With the emergence of lipid nanoparticles, viral vectors, and virus-like particles, therapeutics are entering the clinic that are > 20 nm— much larger than conventional biotherapeutics. For chromatographic separations, the increased size can be countered in two ways. First, the pores of chromatographic stationary phases need to become larger in order for the analytes to enter the pores. This is particularly important for size-exclusion separations because the separation relies on the diffusion in and out of the pores, which is different for differently sized molecules. Second, for separation modes that rely on hydrophobic or electrostatic interactions, such as ion-exchange or hydrophobic interaction chromatography, another approach to cope with bigger molecules is changing to non-porous particles.
Conclusion
Just as molecules evolve, the tools for their analysis do. While these tools are capable of more precisely characterizing novel drug modalities, they often lack robustness and the in-depth understanding necessary to implement them in routine and QC analyses. However, if research funding and time is invested to achieve consistent results with these novel analytic approaches, in parallell with appropriate training, these important tools will find broader application in the future.
References
(1) Lagassé, H. A. D.; Alexaki, A.; Simhadri, V. L.; et al. Recent Advances in (Therapeutic Protein) Drug Development. F1000Res. 2017, 6, 113. DOI: 10.12688/f1000research.9970.1
(2) Stoll, D. R.; Carr, P. W. Two-Dimensional LiquidChromatography: A State of the Art Tutorial. Anal. Chem. 2017, 89 (1), 519–531. DOI: 10.1021/acs.analchem.6b03506
(3) Pirok, B. W. J.; Stoll, D. R.; Schoenmakers, P. J. Recent Developments in Two-Dimensional Liquid Chromatography: Fundamental Improvements for Practical Applications. Anal. Chem. 2019, 91 (1), 240–263. DOI: 10.1021/acs.analchem.8b04841
(4) Buckenmaier, S.; Petersson, P. Dealing with Wandering First-Dimension Peaks in 2D-LC Separations. LCGC North Am. 2022, 40 (5), 201–206. DOI: 10.56530/lcgc.na.me6285m3
(5) Stoll, D. The Future of Method Development for TwoDimensional Liquid Chromatography – Work Smarter, Not Just Harder? LCGC North Am. 2022, 40 (8), 379–382. DOI: 10.56530/lcgc.na.iy5385p1
About the Author
Andrea Krumm studied biotechnology before gaining a Ph.D. in cancer biology. After four years as an applications specialist for optical analytical devices used in life science research, she joined Tosoh Bioscience GmbH in 2020 as a product manager for analytical columns. She is responsible for gathering customer requirements, application areas, and addressing these with new and existing products of the analytical columns line.
Email: andrea.krumm@tosoh.com
Website: www.tosohbioscience.de

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.