Low-Resolution or High-Resolution Mass Spectrometry for Clinical and Forensic Toxicology: Some Considerations from Two Real Cases
How can low-resolution mass spectrometry (LRMS) and high-resolution (HR) MS work together to provide unambiguous results in clinical and forensic toxicology?
The search for xenobiotics in analytical toxicology is based on different screening approaches depending on the context. Targeted analysis is preferred when some toxicants are suspected, but in some cases an untargeted analysis can be performed to detect compounds without preconceptions. Whatever the case, there is a strong need for certainty in the identification of compounds. In this context, the “gold standard” in analytical toxicology is liquid chromatography combined with mass spectrometry (LC–MS). Most of the time, toxicological laboratories are equipped with low-resolution mass spectrometers (LRMS). However, the principle of LRMS is that it can only discriminate compounds by their nominal mass, which can lead to false positives in the case of isobaric components. To solve this relative lack of specificity, high resolution mass spectrometry (HRMS) is necessary to confirm (or deny) LRMS results.
Main Differences Between HR vs. LR
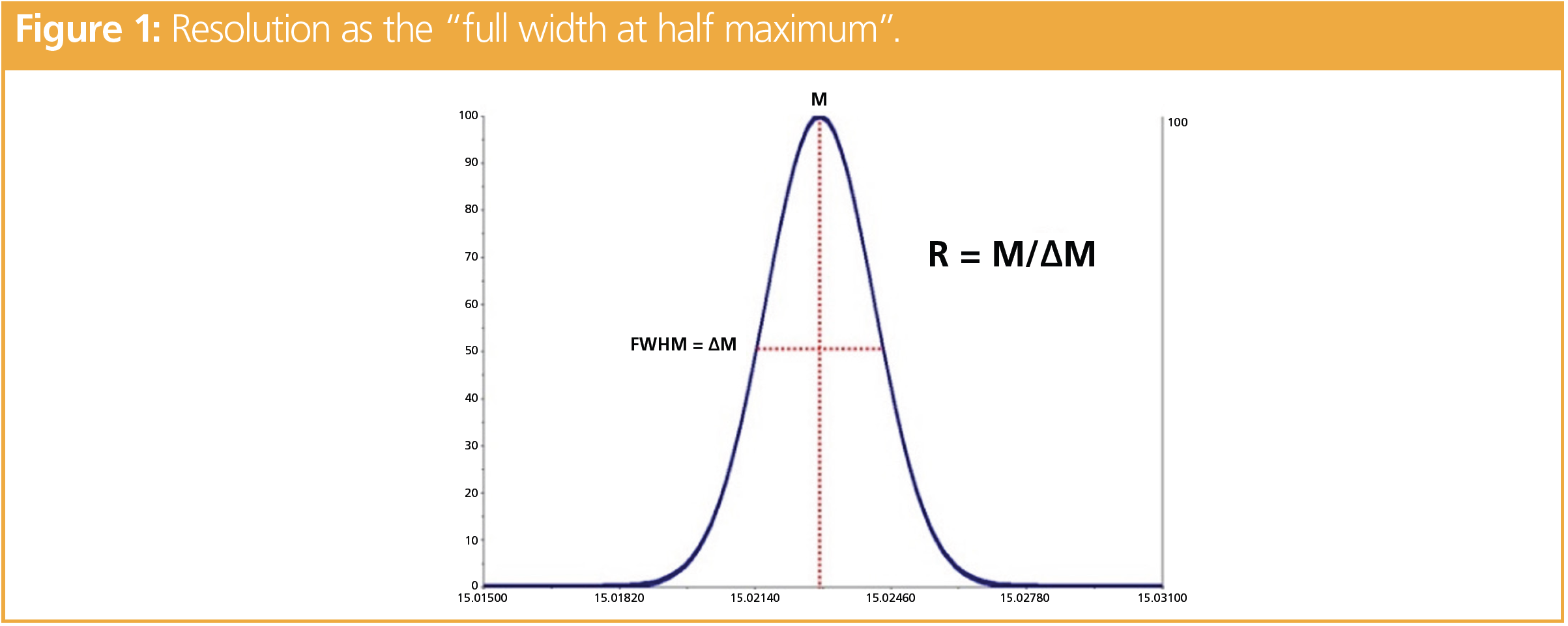
In mass spectrometry, resolution (R) can be defined by the full width at half maximum (FWHM, Figure 1). Mass spectrometers that achieve a resolution of at least 20,000 are called high resolution mass spectrometers (HRMS). The main difference between HRMS and LRMS is the precision in the determination of the mass; the so-called “mass accuracy”. To confirm a compound identification in HRMS, error in the mass determination (Δm) has to be < 5 ppm (equation 1).
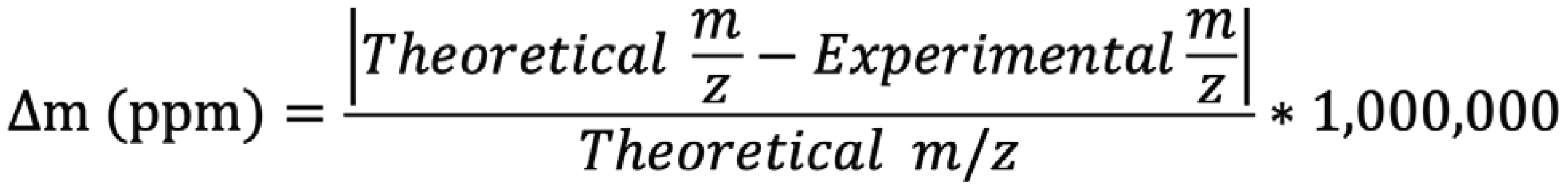
According to this, HRMS systems are known to be able to discriminate compounds by their accurate masses, contrary to LRMS systems, which can only reach a so-called “nominal mass” (± 1 Da) (1).
Roughly, the high selectivity of HRMS ensures certainty in the identification of precursors in MS1 and fragments in MS2, while LRMS cannot differentiate two different compounds when they are isobaric (same nominal masses) and/or share similar fragments and retention times (RT). However, a major drawback of HRMS is that it usually exhibits lower sensitivity and dynamic ranges (2). Consequently, LRMS and HRMS approaches can be seen as complementary tools for laboratories that have to perform screen analysis in multiple contexts. This article presents two real cases that were faced by our forensic unit and for which both LRMS and HRMS approaches were needed to obtain unambiguous results.
The Two Cases
The first analysis concerned a whole blood in a case of driving under the influence of drugs (DUID).
The second case concerned a whole blood analyzed in the context of drug‑facilitated sexual assault (DFSA). In the two cases, the objective of the HRMS analysis was to confirm the presence of compounds detected by LRMS.
Sample Preparation and Chromatographic Separation: In both cases, the following extraction procedure was applied: to 100 μL of whole blood, 200 μL of glacial acetonitrile (-20 °C) was added for sample deproteinization. Then, 40 mg of QuEChERS salts (4 g MgSO4/1 g NaCl/1 g sodium citrate dihydrate/0.5 sodium citrate sesquihydrate) supplied by UCT were added. The mixture was centrifugated and 30 μL of the upper phase was added to 90 μL of aqueous mobile phase and 5 μL was then injected.
For LRMS and HRMS approaches, the chromatographic system consisted of two Shimadzu LC-30 AD pumps (Nexera X2), a CTO 20 AC oven, and a SIL-30 AC-MP autosampler (Shimadzu Corporation). Chromatographic separation was performed on a 100 × 2.1 mm, 2.7‑μm Shim‑Pack Velox biphenyl column (Shimadzu Corporation), using a gradient of (A) ammonium formate 2 mM/0.002% formic acid buffer, and (B) methanol ammonium formate 2 mM/0.002% formic acid buffer as mobile phase at a flow-rate of 0.3 mL/min (0.6 mL/min 11–16.2 min to accelerate column conditioning and equilibration), as follows: 0.00–1.00 min, 5% (B); 1.00–2.00 min, 5 to 40% (B); 2.00–10.50 min, 40 to 100% (B); 10.50–11.00 min, 100% (B); 11.01–13.00 min, 100% (B); 13.00–13.01, 100 to 5% (B); 13.51–18 min, 5% (B). Oven temperature was set at 40 °C.
Mass Spectral Conditions for LRMS and HRMS: A fully validated and routinely employed method allowing the analysis of 48 illicit drugs was used. It was based on a multiple reaction monitoring (MRM) mode with two transitions per molecule. The LRMS system was a triple quadrupole LCMS-8050 (Shimadzu Corporation) (3).
The HRMS system was an LCMS‑9030 (quadrupole time-of-flight [QTOF]) (Shimadzu Corporation). A targeted acquisition mode was used (“MSMS mode”). Briefly, using this acquisition mode, the candidate precursor was searched using full-scan MS1 analysis, while its fragments were secondarily searched using MS2 acquisition after obtaining the exact mass of the precursor.
Case 1: Consumption of Illicit Drugs?
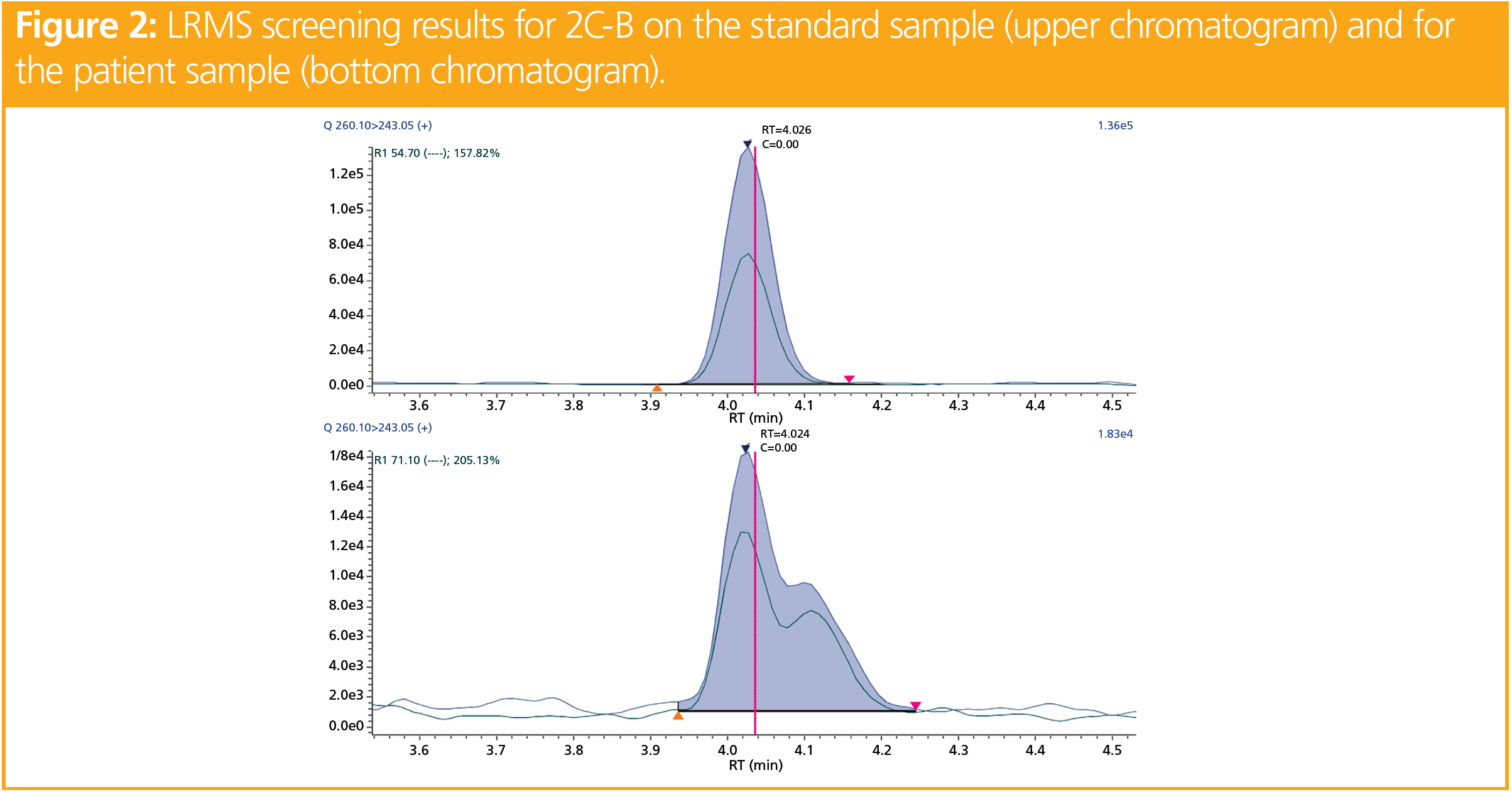
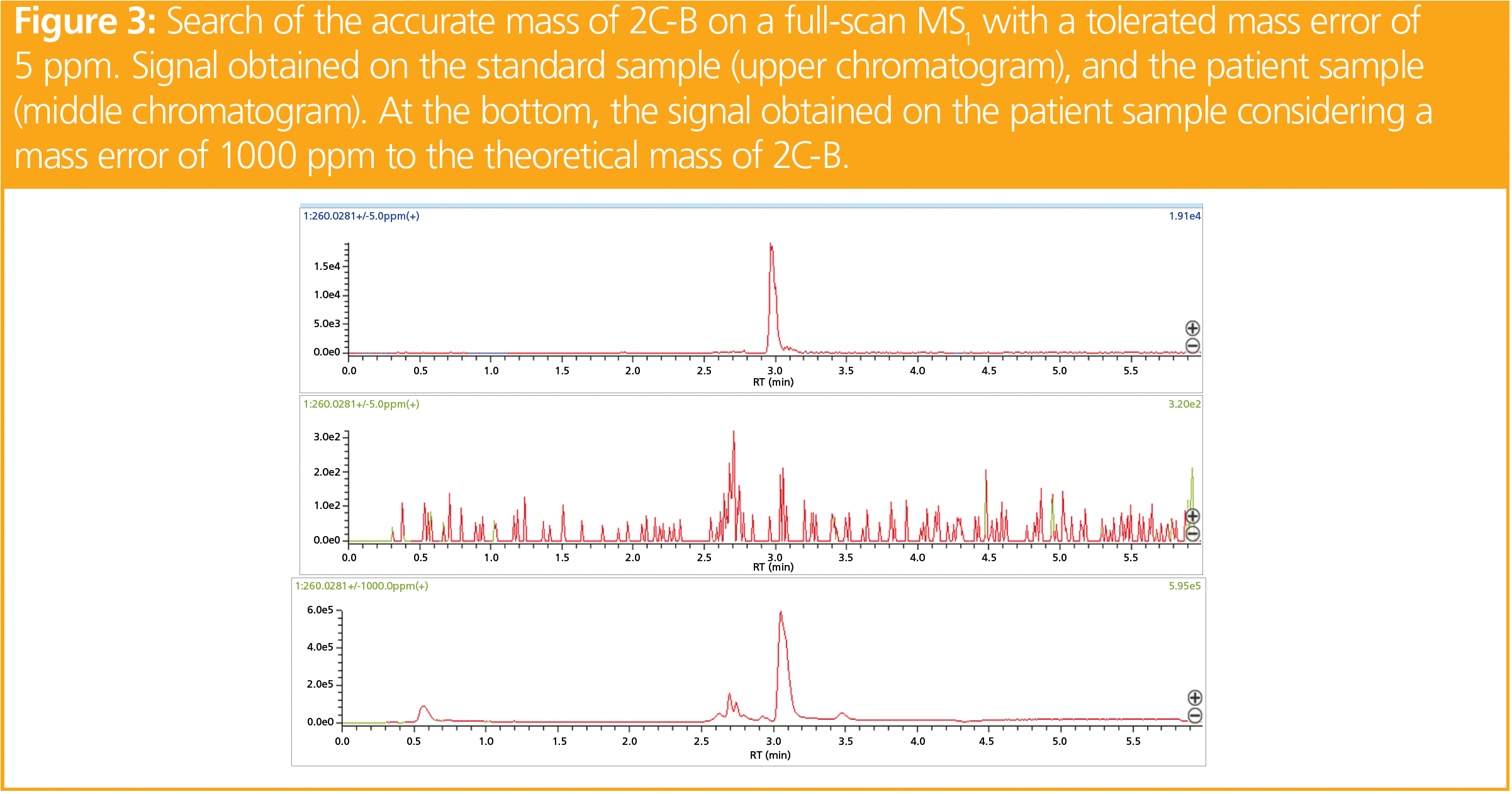
The LRMS targeted acquisition highlighted a signal corresponding to 2C-B, an amphetamine (Figure 2). The RT was consistent with that of our library, and the two transitions (260.10 > 243.05 and 260.10 > 228.10) were detected with similar ratios to those of the standard sample (± 20%). However, 2C-B consumption is not very common in France, and the expert in forensic toxicology asked for a confirmation of its presence in the whole blood of the DUID case by using HRMS (Figure 3). In a whole blood sample spiked at 100 ng/mL of 2C-B, a signal was obtained with a measured mass of 260.02759 m/z (exact mass of 2C-B = 260.0281 m/z; Δm < 2 ppm). When analyzing the whole blood of the driver, a precursor mass of 260.16391 m/z was obtained. Similarly, the fragments obtained in the patients were not those expected (243.13686 m/z vs. 243.0015 m/z; and 228.11419 m/z vs. 227.9780 m/z). The observed signals corresponded to a mass error > 500 ppm for the precursor and the fragments. As mentioned above, Δm < 5 ppm is mandatory to unambiguously identify a molecule. Consequently, the molecule detected by LRMS was not 2C-B.
Case 2: Drug-Facilitated Sexual Assault?
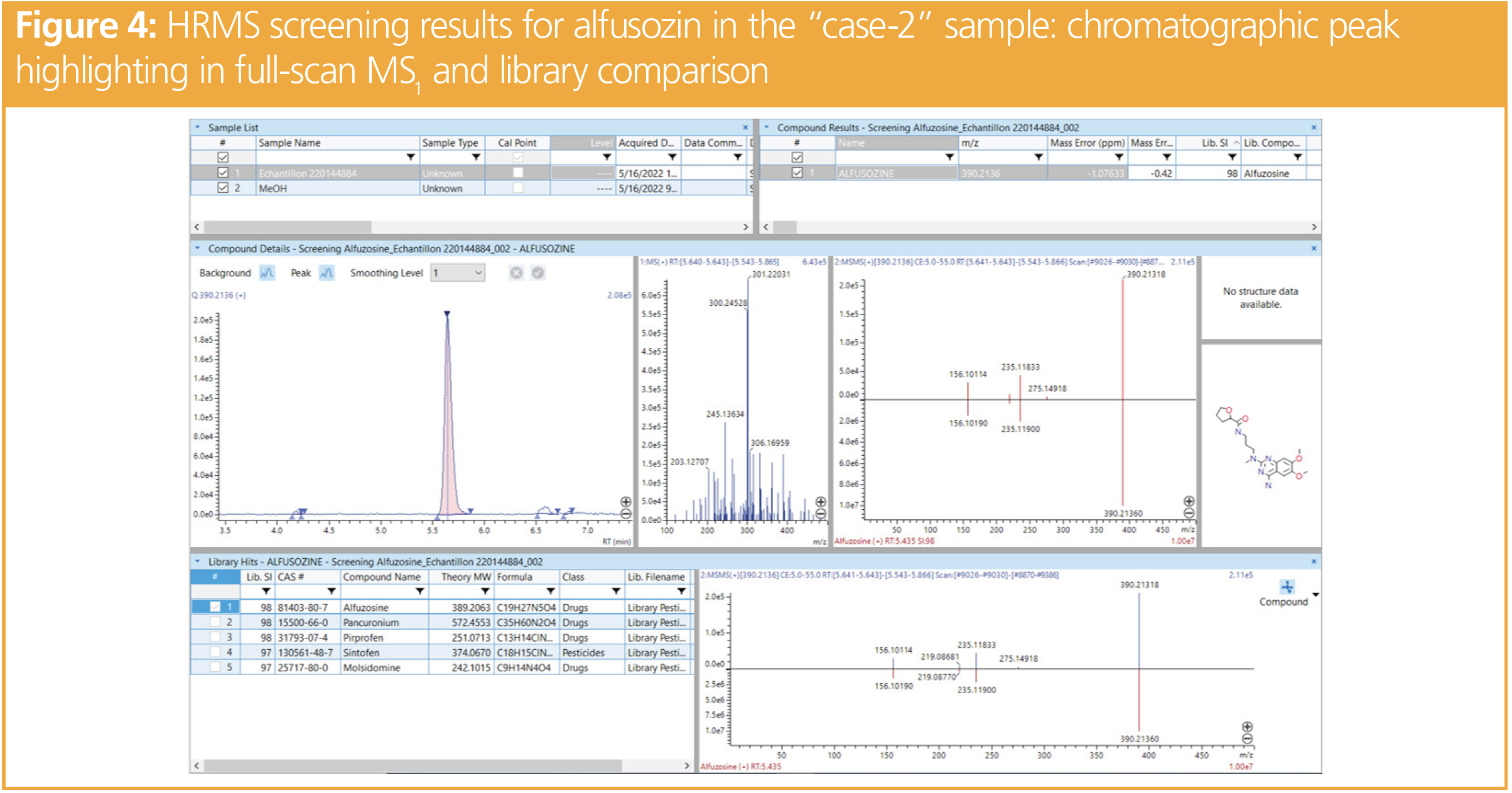
The LRMS screening procedure identified alfuzosin, an alpha-blocker used for functional symptoms of benign prostatic hyperplasia. As the presence of alfuzosin was hardly compatible in the case of a woman and was also not known as a compound employed in DFSA cases, the expert asked for a confirmation using HRMS. The analysis of the whole blood revealed a compound with a mass of 390.21291 m/z, which was favourably compared with the expected mass of alfuzosin (390.2136 m/z Δm < 2 ppm). In addition, fragments were also very close to those expected (235.11833 m/z vs. 235.11900 m/z and 156.10114 m/z vs. 156.10190 m/z). For the detected precursor ion and the fragment, Δm was < 5 ppm, which confirmed the presence of alfuzosin (Figure 4).
Is There a Place for HRMS in Clinical or Forensic Toxicology?
These two real cases, observed in the routine activity of a forensic toxicology unit, illustrate the obvious added-value of using both LRMS and HRMS approaches in a laboratory. LRMS targeted or untargeted screening procedures ensure sensitivity, but these two cases highlighted situations where their specificity could be insufficient. In the first case, isobaric compounds with very similar precursor ions and fragments—which should have led to a false positive result without a secondary analysis by HRMS—were analyzed. In the second case, the detected compound was not consistent with the context and the expert refused to consider the molecule before confirmation by HRMS.
Depending on the laboratory, we believe that HRMS can be used either in combination with LRMS, or as a kind of all-in-one solution, taking into account the recent improvement in the sensitivity and dynamic ranges (4,5).
References
(1) Ojanperä, I.; Kolmonen, M.; Pelander, A. Current use of high-resolution mass spectrometry in drug screening relevant to clinical and forensic toxicology and doping control. Anal. Bioanal. Chem. 2012, 403 (5), 1203–1220. DOI: 10.1007/s00216-012-5726-z
(2) Kaufmann, A. The current role of high-resolution mass spectrometry in food analysis. Anal. Bioanal. Chem. 2012, 403 (5), 1233– 1249. DOI: 10.1007/s00216-011-5629-4
(3) Dulaurent, S.; El Balkhi, S.; Poncelet, L. et al. QuEChERS sample preparation prior to LC-MS/MS determination of opiates, amphetamines, and cocaine metabolites in whole blood. Anal. Bioanal. Chem. 2016, 408 (5), 1467–1474. DOI: 10.1007/s00216-015-9248-3
(4) Grund, B.; Marvin, L.; Rochat, B. Quantitative performance of a quadrupole-Orbitrap-MS in targeted LC–MS determinations of small molecules. J. Pharm. Biomed. Anal. 2016, 124, 48–56. DOI: 10.1016/j.jpba.2016.02.025
(5) Fedorova, G.; Randak, T.; Lindberg, R.H.; Grabic, R. Comparison of the quantitative performance of a Q-Exactive high-resolution mass spectrometer with that of a triple quadrupole tandem mass spectrometer for the analysis of illicit drugs in wastewater. Rapid Comm. Mass Sp. 2013, 27 (15), 1751–1762. DOI: 10.1002/rcm.6628
Elies Zarrouk started a Ph.D. thesis in analytical chemistry in April 2021. The main objective of his work is to develop analytical tools based on a LC–HRMS approach for applications in forensic, clinical, and occupational toxicology. This thesis is supervised by Souleiman El Balkhi (head of the unit of occupational and environmental toxicology, Limoges University Hospital, France) and Franck Saint‑Marcoux (head of the unit of forensic toxicology, Limoges University Hospital). Email: elies.zarrouk@unilim.fr

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.