Ten Common-Sense Corollaries in Pharmaceutical Analysis by High Performance Liquid Chromatography
LCGC Europe
This instalment describes ten corollaries in high performance liquid chromatography (HPLC) and pharmaceutical analysis that most practitioners are likely aware of but may overlook the rationales behind them.
This instalment describes ten corollaries in high performance liquid chromatography (HPLC) and pharmaceutical analysis that most practitioners are likely aware of but may overlook the rationales behind them.
When I started this column five years ago, the goal was to present substantive articles relevant to most high performance liquid chromatography (HPLC) practitioners. The first instalment, “The Essence of Modern HPLC: Advantages, Limitations, Fundamentals, and Opportunities” (published in June 2013), strived to capture some key tenets of HPLC analysis (1). In addition to providing annual reviews of new HPLC products in April issues, I authored a series of white papers on separation science in drug development, and ultrahigh performance liquid chromatography (UHPLC) instruments, benefits, and potential issues, as well as articles on expediting HPLC method development, superficially porous particles, and quality control of biotherapeutics. Other instalments had less conventional titles, such as “Seven Common Faux Pas in HPLC” and “Myths in UHPLC” (2,3).
Today, HPLC is a mature technique with many diverse users, and introducing fresh topics or perspectives on the subject matter is becoming more of a challenge. I am still digging hard to find wide-scoped HPLC topics for discussion, which brings us to this month’s entry. I hope that these viewpoints may bring newer perspectives in the practice of HPLC, even though one may or may not agree with some of the presented rationales for these corollaries.
Ten Common-Sense Corollaries in HPLC and Pharmaceutical Analysis
The goal of most HPLC analyses is to separate analytes from other components in the sample for accurate quantitation. The following list provides practical corollaries along with ramifications that are often overlooked by practitioners:
- HPLC is not complicated, but complex.
- The analytes must be soluble.
- For separation to occur, analytes must be retained and have differential migration rates.
- Use gradient reversedâphase chromatography for stabilityâindicating assays.
- The ultraviolet (UV) detector is the default detector for quality control (QC) of drug substances and drug products.
- The mobile phase controls the separation.
- C18 bonded-phase columns are not all the same.
- The final analyte solutions should be prepared in mobile-phase A.
- There are no perfect HPLC methods.
- LC−mass spectrometry (MS) is a two-dimensional (2D) separation technique.
1. HPLC Is Not Complicated, but Complex
HPLC is not complicated and does not require an advanced scientific degree to understand its core concepts, or excel in its practices. It is, however, complex, because of the scope of equipment components and operating variables (that is, column, mobile phases, pump, autosamplers, detectors, data systems, operating conditions, samples, standards, diluent) working in tandem to generate robust and accurate results (4−6). Differential equations are not needed to understand concepts in isocratic analysis, such as the resolution equation (4,5):
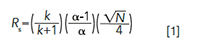
where Rs = resolution, k = retention factor, α = selectivity, and N = plate count.
For gradient elution using reversedâphase chromatography, the linear solvent strength theory is most useful where log k is inversely proportional to the percentage of mobile-phase B (%B) or the organic solvent (7). Gradient elution is complex with more operating factors such as the initial to final %B and gradient time (tG) (4−5,7). Nevertheless, the retention times in reversedâphase chromatography are highly predictable because its mechanism is based primarily on hydrophobic (solvophobic) interactions (4). Other chromatographic modes, such as ion-exchange or mixed-mode chromatography, have more-complex mechanisms and are therefore less predictable.
For this reason, HPLC should not be used if another simpler analytical technique can be used instead. For instance, in regulatory analysis, before sample testing can be performed, the operator needs to perform a system suitability test with an appropriate check standard (for example, 10 injections to verify system precision, sensitivity, and calibration) (5). HPLC analysis may be too onerous for an identification test for an excipient or a starting material, where a simple test by a handheld Raman spectrometer can be completed in a few seconds. Nevertheless, identification by HPLC retention time is always the official method for release testing of drug substances or drug products, because the same analysis is used for potency and impurities determination.
2. The Sample Analytes Must Be Soluble
The greatest strength of HPLC is its amenability to diverse sample analytes ranging from small-molecule drugs, biomolecules, and polymers to inorganic ions (1,5). The motto stands true that “if it is not in solution, it cannot be analyzed by HPLC.” Analyte solubility issues often adversely affect assay outcomes of low-solubility analytes that are difficult to extract from sample matrices. Low recoveries can stem from poor sample preparation steps rather than the HPLC analysis itself.
I recall two potentially outâofâspecification (OOS) investigations that were traced to incomplete extraction of the active ingredients from the drug products. One was a suspension formulation of a low-solubility new chemical entity (NCE) to be used in a clinical trial (5), and another was a sustainedârelease drug product. In both cases, an improved extraction procedure with combined shaking and extended ultrasonication resolved the OOS status.
The quantitative extraction of key analytes from a multicomponent drug product can be very challenging. A case in point is the analysis of waterâsoluble vitamins from a multivitamin tablet where the quantitative extraction of all waterâsoluble vitamins from a single extraction method can be extremely challenging (8).
3. For Separation to Occur, Analytes Must Be Retained and Have Differential Migration in the Column
For HPLC−UV analysis, key analytes must have different retention times to yield accurate quantitation. During HPLC method development, the focus is placed on the physical separation of critical pairs of closely eluted analytes.
For separation to occur, analytes must be retained (that is, they must have a retention factor k > 2, preferably) and have differential migration rates in the column (α or selectivity > 1.0) (5). Separation cannot be achieved without retention; otherwise, a flow injection analysis is performed. The critical pair of analytes must have sufficient differences in the interaction with the stationary phase.
In contrast, baseline resolution is not strictly required in HPLC−MS analyses since specific signals can be customized for each ion according to its mass-to-charge ratio. The exceptions are isobaric isomers whose physical separation is required for quantitation or the detection of a minor component close to the main peak.
4. Use Gradient ReversedâPhase Chromatography for StabilityâIndicating Assays
The stability-indicating assay (purity method) of a drug substance or drug product is the most important analytical methodology in new drug development and production to ascertain the quality of pharmaceuticals (that is, ensuring the safety and efficacy of the product) (9−11).
Have you ever wondered why most stability-indicating assays are based on reversed-phase chromatography? Few purity methods are based on other modes (that is, ion-exchange chromatography, normal-phase chromatography, or hydrophilic interaction chromatography [HILIC]) that have stronger retention mechanisms (polar or ionic interactions). With these stronger retention mechanisms, it is difficult to ascertain that all components in the sample are eluted from the column (that is, achieving total mass balance) (1). Reversed-phase chromatography is mainly based on hydrophobic interactions, a weak dispersive force. With the mobile phase at 100% acetonitrile or methanol, few analytes are retained on the column.
In addition, gradient elution using broad gradients (that is, 5−100% B) is generally used to ensure the retention of the hydrophilic components at the low solvent strength and the elution of highly retained impurities (such as dimers) at high solvent strength. Gradient elution also offers higher peak capacities, enhanced detection sensitivities, and sharper peaks (4–5,7).
5. The UV Detector Is the Default Detector for Quality Control of Drug Substances and Drug Products
The UV detector is the de facto default detector for the quality control of drug substances and drug products by HPLC (5). In most pharmaceutical development and QC laboratories, the standard detector is likely a photodiode-array detector that has similar performance to those of a UV–vis absorbance detector but with the added advantage of providing UV spectral data, and chromatograms, at multiple wavelengths.
The UV detector is the standard detector in pharmaceutical analysis because most drugs are chromophoric and possess UV-absorbing moieties in their structures (such as aromatic rings). Since the entire post-separation HPLC eluent passes through the UV detector flow cell, versus a fraction as in MS, HPLC−UV is capable of very high precision (for example, <0.2% peak area relative standard deviation [RSD]). This high assay precision is needed in release testing of drug substance batches with typical specifications of 98.0% to 102.0% w/w) (9−11). According to International Conference on Harmonization (ICH) guidelines Q3A (R2) and Q3B (R2), the reporting thresholds are 0.05% and 0.10% for impurities and degradation products in drug stubsances and drug products, respectively (12). Although this reporting of impurities is interpreted to mean percent by weight, nevertheless, the UV area percent under the curve is often used during early clinical development before the availability of reference standards for specified impurities (5,9−11). As UV response factors for closely related structures are often similar to the active ingredient, the area percent quantitation for HPLC−UV is usually more reliable than the area percent for LC−MS.
6. The Mobile Phase Controls the Separation in Reversed Phase Chromatography
Where the stationary phase is the medium for analyte retention and interaction in HPLC, the mobile phase controls the overall separation in reversed-phase chromatography. In HPLC method development, efforts typically focus on finding a set of mobile-phase conditions (solvent strength or %B, type of buffer, buffer concentration, pH, organic modifier, and so on) with the appropriate stationary phase for separating all key analytes from other components (5). Fine-tuning the mobile-phase parameters is generally easier since they can be changed continuously and conveniently via pump blending. Exceptions to this rule are sizeâexclusion, chiral, and affinity chromatography where the mobile phase plays a much more minor role.
7. C18 Bonded-Phase Columns Are Not All the Same and Cannot Be Interchanged for Critical Assays
The C18 phase is the most common bonded phase in reversed-phase chromatography applications. It is highly hydrophobic and retentive, and has excellent column batch-to-batch reproducibility. There are hundreds of C18 columns on the market that are based on high-purity silica support materials. These stationary phases, however, can have a different particle size, pore size, particle morphology (totally porous or superficially porous), ligand density, bonding chemistry (mono-, di- ,or trifunctional silane), and endcapping (5).
For critical assays of complex samples, C18 columns are not generally interchangeable and the exact column from a specific manufacturer should be ordered with the exact part numbers. For potency or performance assays of the main component only, a similar C18 bonded column of identical dimension and particle size can often be substituted. Nevertheless, column substitution is generally not allowed in a regulatory method without validation (12). As part of method validation, the effect of replacing the column with columns packed with different batches of stationary phase is evaluated.
8. The Final Analyte Solution Should Be Prepared in MobileâPhase A in Reversed-Phase Chromatography
The final analyte solution, if possible, should be dissolved in the mobileâphase A or diluent of “weaker” elution strength than the starting mobile phase in a gradient analysis (5). Many anomalies such as splitting or fronting peaks are caused by injecting samples dissolved in diluents stronger in elution strength than the starting mobile phase. If a stronger diluent must be used to dissolve the sample, a smaller injection volume (that is, 2–5 µL) should be considered to minimize these problems. It is not unusual to inject a large aqueous sample (for example, 2 mL of drinking water or proteinâbased solutions) directly onto an HPLC system under gradient reversed-phase chromatography conditions because the analytes would be concentrated at the head of the column and eluted according to their hydrophobicities. Peak shape would not be impacted under these circumstances.
9. There Are No Perfect HPLC Methods
I have developed hundreds of HPLC methods for pharmaceutical and other applications and have yet to witness a “perfect” method. Every HPLC analytical method appears to have its caveats, limitations, or pitfalls. An experienced separation scientist can identify these potential pitfalls and find conditions to minimize any issues including those from sample preparation. This situation is particularly true in the development of the first stability-indicating assay for new chemical entities in early clinical studies. One needs a reasonable HPLC method to conduct forced degradation and initial stability studies but typically does not have any reference standards of impurities and degradation products (9–11). The initial method typically undergoes many revisions to accommodate for the presence of new impurities. One recommendation is the use of stageâappropriate method development and validation strategy as proposed by Rasmussen and colleagues to accommodate the rapid process changes during clinical development of new drug candidates (13).
10. LC−MS Is a 2D Separation Technique
2D-LC with high peak capacities is particularly relevant for very complex samples (such as proteomics samples) using two orthogonal separation modes. 2D methods, however, are challenging to develop and implement because of its complexity in equipment and data handling. It should be noted that any LC−MS analysis in the fullâscan mode is a 2D separation since the second dimension is separation by mass-to-charge ratio. Using modern chromatography data systems that excel at integrating UV and MS data, LC−MS data can often be further mined for accurate quantitative determination of each analyte without baseline HPLC resolution. The utility of chemometric software with trending analysis and noise reduction algorithms may further improve the accuracy of quantitative analysis (14–15). This approach, if accepted by regulatory agencies, will significantly improve the efficiency of HPLC method development of stabilityâindicating assays while enhancing method accuracy without the onus of achieving physical separation of all impurities and degradants.
Summary
This instalment describes 10 commonâsense corollaries in the practice of pharmaceutical analysis by HPLC and includes viewpoints on the essence of chromatographic applications, considerations for sample, mode, detection, column, mobile phase, and diluent, and 2D-LC.
Acknowledgements
The author is grateful to Matt Mullaney of Pentec Health, Brian R. Holder of Merck, Giacomo Chiti of Bolton Group B.V., Shula Levin of Bioforum Applied Knowledge, Mangesh Chhadtre of Ashland India, and M. Farooq Wahab of U. Texas Arlington for their reviews of technical content and editorial inputs.
References
- M.W. Dong, LCGC Europe26(7), 393−399 (2013).
- M.W. Dong, LCGC Europe27(8), 415−419 (2014).
- M.W. Dong, LCGC Europe26(11), 637−645 (2013).
- L.R. Snyder and J.J. Kirkland, Introduction to Modern Liquid Chromatography, 3rd Edition (John Wiley & Sons, Hoboken, New Jersey, USA, 2010).
- M.W. Dong, Modern HPLC for Practicing Scientists, (John Wiley & Sons, Hoboken, New Jersey, USA, 2006), Chapters 1−4, 6, 8, and 10.
- D. Guillarme and M.W. Dong. Amer. Pharm. Rev.16(4), 36−43 (2013).
- L.R. Snyder and J.W. Dolan, HighâPerformance Gradient Elution: The Practical Application of the Linear-Solvent-Strength Model, (WileyâInterscience, Hoboken, New Jersey, USA, 2006).
- M.W. Dong and J.L. Pace, LCGC North Am.14(9), 794–803 (1996).
- M.W. Dong, LCGC North Am.33(10), 764–775 (2015).
- D. Kou, L. Wigman, P. Yehl and M.W. Dong, LCGC North Am. 33(12), 900–909 (2015).
- International Conference on Harmonization, ICH Harmonized Tripartite Guideline, Impurities in New Drug Substances, Q3A (R2) and Impurities in New Drug Products, Q3B (R2), (ICH, Geneva, Switzerland, 2006).
- J. Dolan and D. Stoll, LCGC Europe30(6), 292−296 (2017).
- S. Ahuja and M.W. Dong, Eds., Handbook of Pharmaceutical Analysis by HPLC, (Elsevier, Amsterdam, Netherlands, 2005), pp. 145−190.
- M.W. Dong, LCGC Europe31(4), 212–221 (2018).
- Online Chemical Analysis System (OCAS): ocas.chemopower.com/
Michael W. Dong is a principal of MWD Consulting, which provides training and consulting services in HPLC and UHPLC, method improvements, pharmaceutical analysis, and drug quality. He was formerly a Senior Scientist at Genentech, Research Fellow at Purdue Pharma, and Senior Staff Scientist at Applied Biosystems/PerkinElmer. He holds a PhD in Analytical Chemistry from City University of New York, USA. He has more than 100 publications and a best-selling book in chromatography. He is an editorial advisory board member of LCGC North America and the Chinese American Chromatography Association. Direct correspondence to: LCGCedit@ubm.com

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.











