Targeting Specific Matrix Interferences for Sample Preparation
This article describes the evolution of traditional solid-phase extraction (SPE) into new techniques that are customized to the characteristics of specific matrices, including the removal of β-glucuronidase from urine samples and the removal of phospholipids and proteins from plasma samples.
Photo Credit: Oewsiri/Shutterstock.com

Matt Brusius, Phenomenex, Torrance, California, USA
This article describes the evolution of traditional solid-phase extraction (SPE) into new techniques that are customized to the characteristics of specific matrices, including the removal of β-glucuronidase from urine samples and the removal of phospholipids and proteins from plasma samples.
Sample preparation is a critical component of all chromatography, whether it is small molecule work in clinical research, drug discovery in the bioanalytical space, or even in the nascent cannabinoid testing industry. Sample preparation has a profound effect on laboratory productivity. Though large amounts of time can be spent preparing samples compared to the actual analysis time, proper sample preparation can greatly reduce the overall processing time and can even reduce system maintenance. As laboratories face pressures to reduce time requirements and costs, sample preparation has evolved from traditional solid-phase extraction (SPE) to accommodate these needs without completely compromising the cleanliness of the resulting sample. In this article we will discuss two of these newly evolved cleanup approaches that use chemical filters–β-glucuronidase removal from urine samples and the removal of phospholipids and proteins from plasma samples.
Solid-Phase Extraction
Solid-phase extraction (SPE) is one of the techniques available to bridge the gap between sample collection and analysis. The purpose of performing SPE is threefold: cleanup, concentration, and solvent exchange (1).
Traditional SPE follows a “catch and release” protocol. That is, the SPE sorbent is chosen specifically to target analytes of interest in an effort to retain them from a given sample matrix. This is followed by a washing step that removes unwanted interferences.
The final step in the SPE process is elution, which releases the analytes from the sorbent. The elution solvent usually has a high organic percentage or has been pH modified and is typically smaller in volume than the starting sample, thereby functioning to concentrate the sample.
By choosing an elution solvent that is appropriate for the analysis, such as a volatile organic solvent for gas chromatography (GC) analysis, or an aqueous or methanol solvent for reversed-phase high performance liquid chromatography (HPLC), one can create an effective solvent exchange system that can provide a more streamlined, accurate, and tailored workflow compared to traditional liquid–liquid extraction (LLE). While traditional SPE is still the “gold standard” in the industry, method development can be cumbersome and multiple steps are required. Advances in instrumentation sensitivity and the ability to achieve low detection levels have also reduced the need for sample concentration by SPE, allowing analysts to inject more dilute samples without significantly compromising their detection limits.
Targeting Specific Matrix Interferences
Recently, sample preparation techniques have been developed that target and remove specific matrix interferences as opposed to analytes of interest, which is the case with traditional SPE. These interference-specific products deliver samples that are clean enough, while mitigating the complexity of method development and reducing the amount of time spent preparing samples. It should be noted that these techniques are targeted towards specific interferences and may not address all interferences that may be present in each unique sample matrix.
Sample matrix varies by industry. In clinical research and forensic toxicology, urine is the most common, although the use of oral fluids is on the rise. From a sample preparation perspective, urine poses a unique challenge because it requires the use of the enzyme β-glucuronidase to perform a hydrolysis step to free the urinary metabolites of their glucuronide moiety. β-glucuronidase is a large 384 kDa tetramer that can precipitate out on LC columns at high concentrations of organic mobile phase and cause column lifetime issues. While “dilute-and-shoot” methods can help to mitigate the amount of protein (β-glucuronidase) in each injection on the LC column and mass spectrometer (5), this practice provides a 10- to 20âfold reduction in sensitivity, which can be problematic for notoriously low-responding compounds, such as buprenorphine and norbuprenorphine.
An ideal sample preparation method in this space is the use of a chemical filter that removes the β-glucuronidase enzyme while preserving the recovery of analytes of interest and maintaining sensitivity of analysis.
Method Specifics and Evaluation of β-Glucuronidase Removal Procedure
Method: To test the effectiveness of the β-glucuronidase removal procedure, we compared the resulting eluent to a sample prepared with “dilute-and-shoot”. The procedure for β-glucuronidase removal was performed with a 96-well plate containing sorbent specifically designed to remove the β-glucuronidase enzyme. A 200-μL measure of the urine hydrolysate solution was diluted with 135 μL of methanol and 0.1% formic acid and loaded onto the β-glucuronidase removal plate. The wells were evacuated using 5” Hg on a vacuum manifold. The passâthrough was collected and directly injected for analysis on an LC–tandem mass spectrometry (MS/MS) system.
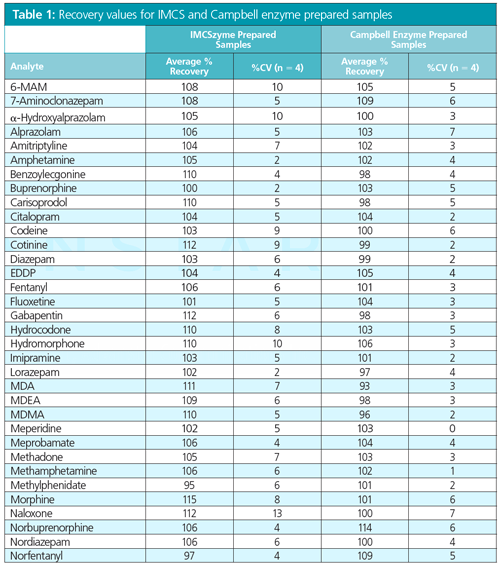
Results: Absolute recovery was the first metric determined for assessing the performance of this technique. Table 1 shows a large panel of drugs of abuse with recovery values >90%, indicating that the sorbent provides consistently high analyte recoveries.
To better understand the magnitude of protein removal using the β-glucuronidase removal method, a Bradford assay with coomassie blue dye was conducted. The Bradford assay uses a brilliant blue G-250 dye whose aromatic moieties and di-sulfonic acid groups bind to the protein’s basic and aromatic sidechains, which produce a protein dye complex that is easily monitored using a spectrophotometer at λ = 595 nm.
The purpose of this relatively simple experiment was to first determine the baseline content of protein found in a hydrolyzed urine solution and then to assess the percentage of protein removed by the β-glucuronidase removal filter method.
In accordance with this, the standard curve was generated (not shown). Based on this curve the pre-filtered enzyme urine mix was determined to have 1604 µg/mL of protein content (actual protein content of sample, 1670 µg/mL), while the sample post-filtration showed 224 μg/mL of total protein, indicating an 86% reduction in protein present in the sample.
The β-glucuronidase removal method provides acceptable absolute recoveries for all drugs of abuse tested while removing 86% of the protein (enzyme) present in the sample. Because this technique avoids large dilutions, these samples provide greater sensitivity for all compounds compared to a standard 10× “diluteâandâshoot” protocol as highlighted in Figure 1, which shows comparative response for norbuprenorphine in a sample that has been prepared by β-glucuronidase removal filtration to one that has been diluted 10×.
Bioanalytical Pharma Transition
The matrix used in pharmaceutical ADME and DMPK analyses is usually plasma. In comparison, the protein content of human plasma is reported to be approximately 70 mg/mL, which is 40× more protein content than what was reported using the Bradford assay for the enzyme–urine solution (2). This significantly larger protein content combined with what is generally considered to be a more complex matrix means that sample preparation is absolutely required for plasma samples.
Historically, scientists involved in small molecule bioanalytical work have sought to simplify the sample preparation process. SPE has been seen as a last resort because it is time-consuming and requires an intensive method development process. For these reasons, less selective techniques like protein precipitation (PPT) and LLE are often preferred.
Protein precipitation is an effective sample preparation method, but it sometimes yields insufficient cleanup. LLE can be cumbersome and can lead to disadvantages such as the formation of emulsions, the use of caustic waterâimmiscible solvent, and a tedious process that is challenging to automate. These challenges have led to the search for another solution to provide a cleaner protein precipitation and remove phospholipids.
A Relatively New Approach to Phospholipid Removal
Phospholipids are the major component of all cell membranes and are comprised of a permanently charged head group with long chain non-polar fatty acid tail(s). It is the extremely hydrophobic nature of these phospholipids that causes them to build up in HPLC columns over time and can lead to premature column death. Traditional protein precipitation does not remove phospholipids. In addition to column lifetime issues, phospholipids are notorious for causing ion suppression in ESI mode MS and, depending on retention time of analytes, they can completely suppress signal.
We will examine how a phospholipid removal method to remove both protein and phospholipids using a simple organic precipitation protocol can improve downstream analysis.
Method: To compare the phospholipid removal method and cleanup, two sets of samples were analyzed: one using a standard protein precipitation filter plate, the other using a filter plate with phospholipid removal sorbent.
To both sets of samples, 750 μL of acetonitrile with 1% formic acid was added to the filter plate followed by 250 μL of plasma. The plate was then vortexed for 2 min to facilitate a protein crash, followed by the application of vacuum at 5” Hg to pull sample through the plate. Samples were then dried down and reconstituted in mobile phase in preparation for ESI+ LC–MS/MS analysis
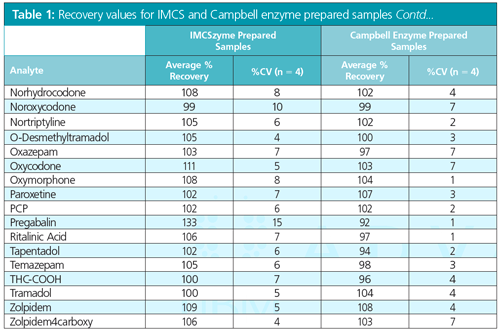
Results: We can monitor for the presence of phospholipids by using the 184–184 transition of the MS, which targets the head group fragment of phospholipids. In Figure 2, a post-column infusion study is performed using amoxapine. The trace on the left represents the protein precipitated plasma sample, while the one on the right shows protein precipitated plasma that has undergone phospholipid removal. It is important to note that the trace on the right shows no suppression zones that correlate to phospholipid elution.
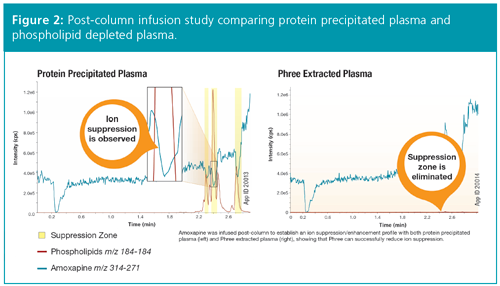
In addition, a column sensitivity study was performed on both types of plasma, this time using diclofenac as the probe. Figure 3 shows an immediate reduction in sensitivity for the protein precipitated plasma and a steeper slope in the reduction of sensitivity over 250 injections in comparison to plasma that underwent a phospholipid removal step. This indicates that phospholipids are building up over time and negatively affecting peak area response for diclofenac.
Protein precipitation with phospholipid removal is a unique chemical filter platform for scientists analyzing small molecules in plasma samples, offering advantages over LLE and protein precipitation. Phospholipid removal can protect from ion suppression issues that are sometimes present with ESI-MS and can help maintain method sensitivity in a workflow that is just as easy and quick as organic-induced protein precipitation.
Conclusion
This article focuses on small-molecule analysis using a sample preparation technique that removes proteins which could interfere with the analysis. But what about the analysts who are interested in analyzing these proteins? And what about biologics in general?
Biologics refers to anything made by the body, and research usually focuses on some aspect of proteins, peptides, oligonucleotides, or amino acids. In fact, biologics constitutes an increasingly larger portion of new therapeutics-up to 40% of some company’s clinical stage pipelines (3).
The trend in sample preparation over the next decade will seek to find a balance between techniques that remove proteins which interfere with small molecules, and techniques that enable the analysis of intact proteins (top-down proteomics), or the peptides that compose those proteins (bottom-up proteomics).
In the latter case, the use of spin filters in bottom-up proteomics has many similarities to traditional SPE. This type of work replaces a sorbent of specific chemistry with a particular molecular weight cutoff filter. When protein–surfactant-containing solution is loaded onto the spin column, the proteins are captured and effectively concentrated (the catch step in SPE). Low-molecular-weight interferences and salts pass through the filter, and buffer exchange and alkylation ensues to remove contaminants and to prepare for elution (wash). Finally, the peptide fragments are eluted with a trypsin digestion and centrifugation (release). Organic extraction of the eluent produces peptides that are pure and ready for analysis via LC–MS (4).
While it seems clear that sample preparation methods will diversify and improve over the coming years, traditional methods, such as SPE, still hold advantages over other techniques because they can concentrate, clean up, and solvent switch. However, as discussed above, they do require method development and a lengthy processing time. Newer interference-targeted filter products and methods have become more popular because of their ease of use and simplicity. However, these are targeted towards specific interferences and may not address all interferences that may be present in each unique sample matrix. Finally, as drug discovery is pushed deeper into biologics, sample preparation will become more specific and in some cases more time-consuming. Determining the right balance between cleanup and simplicity will ultimately be determined
by the sample matrix and the overall goals of the analysis.
References
- Nigel J.K. Simpson, Solid-Phase Extraction: Principles, Techniques and Applications (Marcel Dekker, New York, USA, 2000).
- Y. Shen, J. Kim, E.F. Strittmatter, et al., Proteomics 5, 4034–4045 (2005).
- A. Gautam and X. Pan, Drug Discovery Today 21(3), 379–384 (2016).
- P. Feist and A.B. Hummon, Int. J. Mol. Sci.16, 3537–3563 (2015).
- J.C. Kissack, K.C. Lee, and K.E. Moeller, Mayo Clinical Proceedings83(1), 66–76 (2008). http://www.fmed.uba.ar/depto/toxico1/articulos/23.pdf
Matt Brusius is the Product Manager of sample preparation at Phenomenex, Torrance, California, USA.
E-mail:MatthewB@phenomenex.comWebsite:www.phenomenex.com

Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.
The Effect of Time and Tide On PFAS Concentrations in Estuaries
April 8th 2025Oliver Jones and Navneet Singh from RMIT University, Melbourne, Australia discuss a recent study they conducted to investigate the relationship between tidal cycles and PFAS concentrations in estuarine systems, and offer practical advice on the sample preparation and LC–MS/MS techniques they used to achieve the best results.














