Suspect Screening of Chemicals in Food Packaging Plastic Film by Comprehensive Two-Dimensional Gas Chromatography Coupled to Time-of-Flight Mass Spectrometry
LCGC North America
The occurrence of additives like plasticizers in plastic food packaging has been documented, but information on their potential migration into foods is limited. In this pilot study, 2D GC–MS was used to identify chemicals extracted from a common stretch plastic film with a series of organic solvents. In total, 91 compounds were tentatively identified, including five PCBs.
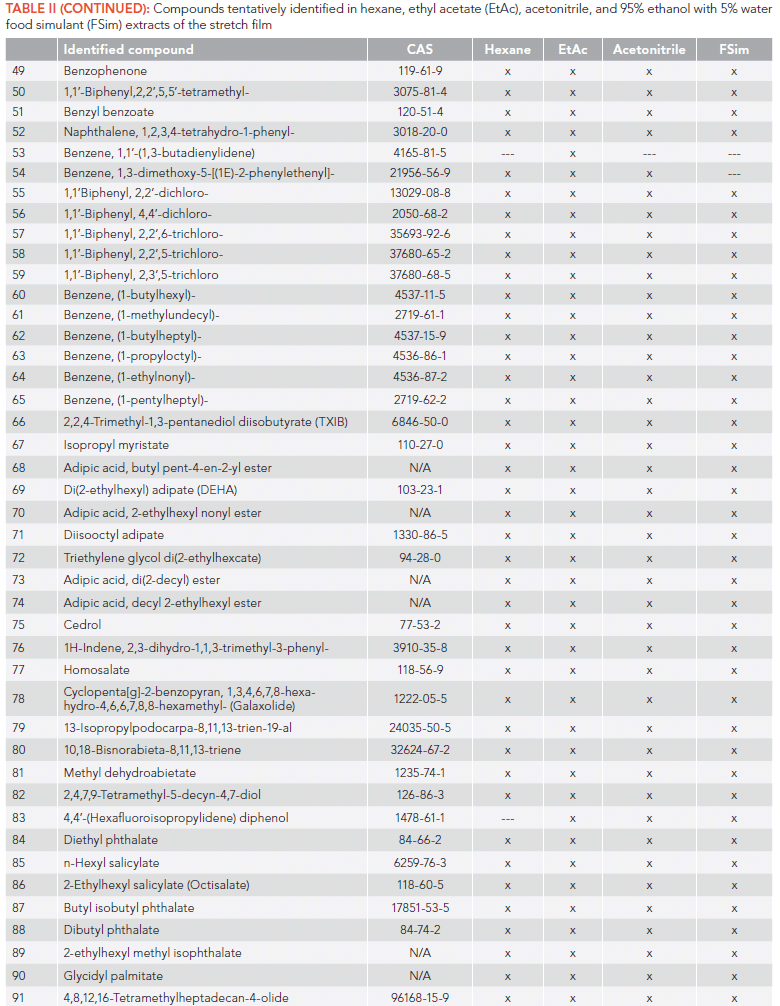
In this study, chemicals extracted from a food packaging plastic film with a series of organic solvents were tentatively identified with comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC–TOF–MS). A total of 91 compounds were tentatively identified in the film extracts, based on high mass spectral matching with the US National Institute of Standards and Technology (NIST) mass spectral (MS) library. The tentatively identified chemicals included common plasticizers: di(2-ethylhexyl) adipate (DEHA), diethyl and dibutyl phthalates; polymer and plastic additives: hexafluorobisphenol A (bisphenol AF), 2,4,7,9-tetramethyl-5-decyn-4,7-diol (TMDD), methyl dehydroabietate, and 2,2,4-trimethyl-1,3-pentanediol diisobutyrate (TXIB); ultraviolet (UV) filters: homosalate, 2-ethyhexyl salicylate (octisalate); fragrances: cedrol and galaxolide, flavoring agents: n-hexyl salicylate and benzophenone. Additionally, representatives of polycyclic aromatic hydrocarbons (PAHs), alkylated naphthalenes, linear alkylbenzenes, and phenyl, biphenyl, and diphenyl compounds were identified. Unexpected discovery of low chlorinated polychlorinated biphenyls (PCBs) in the film extracts suggests their source should be investigated further. The tentatively identified compounds were characterized based on use, source, chemical properties, and previously reported occurrences in plastic food packaging materials, food, or the environment.
Food packaging is important in protecting food, extending its shelf-life, and providing consumers with food handling convenience. However, during storage and handling, chemicals from food packaging materials may potentially migrate into packaged food. The important food safety questions are: Do chemicals leach from food packaging materials when they come in contact with food? If so, at what rates? Does this present any risk to consumers? The lack of answers indicates a knowledge gap and the evident need for research in this area.
Among multiple types of food packaging (such as plastic containers, paper packaging, bottles), plastic films are the most complex in structure and composition, because they are constructed of multiple laminate layers with additives in between to attain the desired properties. Stretch plastic films, also known as cling films, are one of the most widely used food contact materials in both commercial and consumer applications. The global market for flexible plastic packaging reached 11.3 million tons in 2009, continuing to increase at 4% per year to 2016 (1). In terms of composition, polyethylene and polypropylene polymeric materials are most commonly used in plastic films, and other polymers such as polyethylene terephthalate (PET), polyvinyl chloride (PVC), and polyamide (nylon) are used less frequently. These plastic polymers are generally inert, but chemicals added to polymeric films to improve their properties (such as antioxidants, light stabilizers, plasticizers, thermal stabilizers, lubricants, and antistatic agents), plus non-intentionally added substances referred to as NIAS (chemical impurities, contaminants, and chemicals formed during degradation or reaction of added chemicals) may migrate into packaged food. The occurrence of food packaging additives: antioxidants, ultraviolet (UV) stabilizers, and plasticizers (2); print-related contaminants (3); phthalates, polycyclic aromatic hydrocarbons (PAHs), photoinitiators, bisphenols, and polyfluorinated compounds (4) in plastic and paper food packaging has been previously documented. However, there is limited information on their potential migration in foods. A recent review outlined analytical challenges in identifying NIAS from food packaging materials (5), including lack of information on the composition of materials and added ingredients, complex structures, and the need for sophisticated analytical techniques. Recently, nontargeted studies utilizing high resolution mass spectrometry (HRMS) were conducted to identify compounds in polycarbonate food contact plastics (6) and nano-films (7).
The goal of this pilot study was to perform a comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC–TOF-MS) nontargeted qualitative screening to identify and characterize compounds extracted from food packaging stretch film into FDA-approved food simulant and organic solvents with different polarities. We sought to create a list of potential migrants for future studies on migration of chemicals into fatty foods, particularly ground beef.
Experimental
A commonly used stretch plastic film for food packaging, acquired from a local store, was sampled and extracted with US FDA approved food simulant: water–ethanol, 5%:95%, respectively and with three organic solvents with different polarities: hexane and ethyl acetate, mimicking lipophilic properties of fat-containing foods, and with acetonitrile, making it possible to extract both polar and relatively nonpolar chemicals. Approximately 4 g of film samples were completely immersed and extracted with 40 mL of solvents in thoroughly solvent-rinsed glass beakers using ultrasonic extraction for 30 min. Reagent blanks consisting of the representative solvents were processed alongside the samples to account for possible laboratory contamination from glassware, solvents, and laboratory equipment.
In the next experiment, we exposed ground beef samples, acquired from a local supermarket, and previously not exposed to any food packaging, to the plastic film: 1) during 2 min microwave defrosting of a frozen beef samples, and 2) during microwaving of non-frozen beef samples for 30 s (high power). After exposure, the beef samples were extracted with hexane at 1 g/mL ratio. Control beef samples (not covered with plastic film) were processed along with the exposed beef samples.
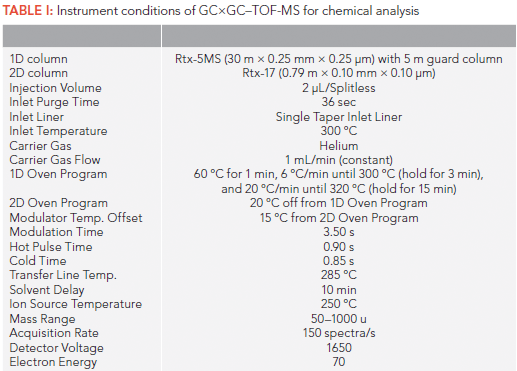
The extracts of the plastic film and ground beef were analyzed using a LECO Pegasus 4D GC×GC–TOF-MS to identify extracted compounds. The instrumental conditions are summarized in Table I. The data generated by GC×GC–TOF-MS was processed by LECO ChromaTOF software (version 4.50.8.0 optimized for Pegasus). Peaks were searched at S/N = 100 or above, and then, an automatic library search was conducted to find most similar mass spectrum for each detected compound in the 2014 National Institute of Standards and Technology (NIST) electron impact (EI) mass spectral library. Compounds with a similarity score of 700 or above (maximum score = 999) were selected, and the selected compounds were further manually reviewed for their similarity with matching mass spectra from the NIST EI mass spectral library. Multiple ions (n > 3) or unique features must be present for comparison. For example, phthalates contain mass (m/z) 149 at high abundance, and aromatic or cyclic compounds or halogenated compounds have unique patterns of mass fragments or isotope ratio patterns. After manual review, highly identifiable compounds were selected. The selected compounds were checked for their presence in each corresponding procedural blank sample. For final inclusion, the peak should not be present in the corresponding control sample. Otherwise, the peak area (abundance) must be 10 times greater than the corresponding peak area in the control sample. Data analysis for the beef extracts was conducted similarly, with the only difference in the inclusion criterion which was a ratio of three between peak abundance for exposed beef sample versus control. It should be noted that the compounds reported in this paper are identified tentatively, since they were not confirmed with their authentic standards, except 2,2',5-trichlorobiphenyl.
Results and Discussion
In the USA, food contact materials are regulated by the US Food and Drug Administration (FDA) under the Food, Drug, and Cosmetic Act. Title 21 of the Code of Federal Regulations (21 CFR) provides regulations for substances that may be safely used as "indirect food additives," generally recognized as safe (GRAS) and prior-sanctioned substances used under conditions of good manufacturing practice (GMP). Indirect food additives are substances in food packaging materials that may come in indirect contact with food.
In this study, we selected an FDA recommended food simulant for fatty foods (95% ethanol: 5% water, v/v) and organic solvents used for GC analysis (hexane, ethyl acetate, acetonitrile) for extracting organic chemicals from the stretch film. It was previously demonstrated that amounts of plasticizers, including adipates, citrates, and phthalates, were in good agreement when packaging films were extracted with ethyl acetate in Soxhlet extraction versus with 96% ethanol food simulant according to the European Union (EU) regulatory rules on migration experiments (8). Additionally, there was no difference in amount of di(2-ethylhexyl) adipate (DEHA) migrated from films into 96% ethanol versus iso-octane.
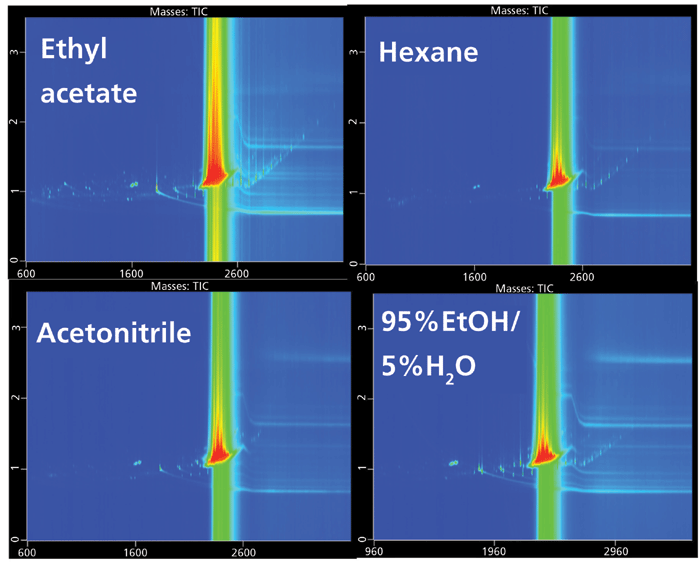
The numbers of tentatively identified chemicals were 91 in ethyl acetate, 74 in acetonitrile and hexane, and 64 in ethanol–water extracts (Figure 1). Other migrated chemicals were not selected for identification due to their low mass spectral matching with the NIST database. The identified compounds with their CAS numbers and occurrence are presented in Table II. Most of the identified chemicals were extracted from plastic films into all studied solvents. However, some migrated selectively into specific solvents. Thus, 14 compounds were identified in ethyl acetate extracts, but not found in other solvent extracts (Table II). Two chemicals, benzene-1,3-bis(acetyl) and 9,10-dihydrophenanthrene (Table II, #14 and 37), were found in hexane and ethyl acetate extracts only. Both chemicals are practically insoluble in water, and therefore most likely only dissolve and migrate to the least nonpolar solvents during extraction. Another interesting example of selective migration was benzoic acid, which was identified in ethyl acetate and acetonitrile extracts, but not in ethanol-based food simulant and hexane. Benzoic acid is used as a food preservative, E210–E213. Lastly, 1,2,3,10b-tetrahydrofluoranthene was identified in ethyl acetate and ethanol–water extracts only, demonstrating a differential pattern of migration.
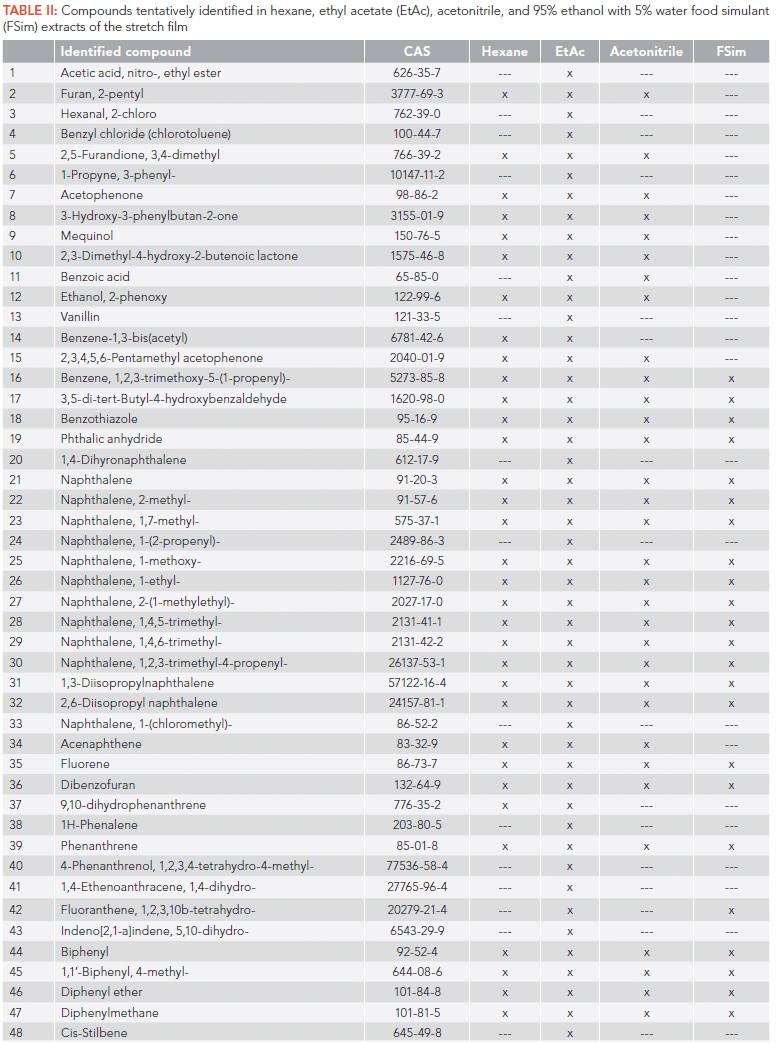
Overall, several classes of chemicals were identified among the extracted compounds: alkylated naphthalenes used as lubricants; polycyclic aromatic hydrocarbons (PAHs) including those on the US Environmental Protection Agency (EPA) priority pollutant list; plasticizers, polymer additives, UV filters, flavoring agents and fragrances, surfactants, adhesives, products of thermal degradation, and polychlorinated biphenyls (PCBs).
Alkylated Naphthalenes
Several alkylated naphthalenes (Table II) were identified in the extracts. These compounds are used as lubricants in many applications, including plastic film manufacturing. Migration of alkylated naphthalenes into food simulants and organic solvents has been previously reported for plastic baby bottles (9) and food contact paper (4). Among alkylated naphthalenes, 2,6-diisopropyl naphthalene was the most frequently identified compound leaching from food contact materials reported by others (4,9–11). Interestingly, this chemical is intended for use in the manufacturing of pesticides as a plant growth regulator. In our residual analysis of pesticides, 2,6-diisopropyl naphthalene is often detected in reagent blanks when plastic labware is used.
PAHs
Another class of extracted substances were PAHs, and 11 compounds were identified: naphthalene, acenaphthene, fluorene, phenanthrene, dibenzofuran, 9,10-dihydrophenanthrene, 1H-phenalene, 1,2,3,4-tetrahydro-4-methyl-4-phenanthrenol, 1,4-dihydro-1,4-ethenoanthracene, 1,2,3,10b-tetrahydro-fluoranthene, and 5,10-dihydroindeno[2,1-a]indene. Naphthalene, acenaphthene, fluorene, and phenanthrene are included on the US list of priority PAHs of frequently found in environmental samples, but not on the EU priority list based on toxicity (12). In a study of quantitative migration of these four PAHs from food contact paper, no acenaphthene was found, while the measured levels of phenanthrene were the highest, followed by fluorene and naphthalene (4). On the other hand, naphthalene was the most frequently identified compound migrating from various food contact materials into food simulants, food, or organic solvents. For example, naphthalene was reported in milk drinks contained in low density polyethylene (LDPE) bottles, and its migration levels increased with storage time and milk fat content (13). Similarly, naphthalene was shown to migrate into food simulants from baby bottles made of polypropylene, polyamide, and silicone (9,11).
Plasticizers
As expected, various plasticizers, including adipates and phthalates, were identified in film extracts. Plasticizers are added to polymers in plastic materials, including stretch films, to achieve the desired properties, such as stretching, flexibility, and durability. According to a migration study on food packaging films, plasticizers comprise 3 to 10% of the films (w/w) (8). The most common plasticizers are phthalate- and adipate-based compounds. Plasticizer migration generally occurs at their direct contact with foods, and is shown to be greater for fatty foods and increased temperatures (14). The most notable identified adipate-based plasticizers were diisooctyl adipate and di(2-ethylhexyl) adipate (DEHA). Both are approved by the US FDA for use in food contact substances as indirect additives (15), with DEHA approved for use only as a components of adhesives. Five adipic acid esters were also identified in the extracts. Diethyl and dibutyl phthalates are commonly used plasticizers, and both are included in the US FDA indirect additives for food contact substances. Dibutyl phthalate was banned in the EU for use in cosmetics, and restricted for use in children's toys in the EU and the USA. In a study on occurrence of common plasticizers, including DEHA and diethyl and dibutyl phthalates in Canadian food, only DEHA was detected in cling films and packaged cheese, beef, pork, chicken, and fish samples (16). Similarly, DEHA was reported migrating into cheese samples from food grade film (17). Dibutyl- and diethyl phthalates were also detected leaching from food contact paper (4) and dibutyl phthalate from polypropylene baby bottles (9).
Fragrances and Flavoring Agents
Several fragrance and flavoring additives were identified in the extracts. For example, n-hexyl salicylate is a food additive flavoring agent (EU Food Improvement Agents) and an odor agent for fragrance. It is also widely used in personal care products, laundry and dishwashing, air freshening and cleaning products. Cedrol is a flavoring agent and food additive (EU Food Improvement Agents); it was also detected in leachate from silicone baby bottles (9). Another flavoring agent, benzophenone, is approved as a food additive in the EU. Additionally, it is used in adhesives and sealants, paints, inks, toners, and colorant products, and personal care products.
Musk galaxolide is used as a fragrance in many consumer products, including cleaning, laundry and dishwashing products, personal care products and plastic. Galaxolide was identified in post-consumer plastic packaging waste in Germany (18), but, surprisingly, this was the only study reporting its occurrence.
UV Filters
Two common UV filters were identified in the film extracts: 2-ethylhexyl salicylate (octisalate) and homosalate. Both are widely used in sunscreens and cosmetics as sunblocks. Many UV filters are added to plastic films to protect against sun exposure; oxybenzone, for example, is approved by US FDA as an indirect food additive.
Plastic and Polymer Additives Several plastic and polymer additives, including hexafluorobisphenol A, 2,4,7,9-tetramethyl-5-decyn-4,7-diol (TMDD), methyl dehydroabietate, and 2,2,4-trimethyl-1,3-pentanediol diisobutyrate (TXIB), were identified. Hexafluorobisphenol A, also known as bisphenol AF (BPAF), is an alternative to bisphenol A, and is used in industrial applications of polymers and synthetic rubber, among others. TMDD is used as non-ionic surfactant, adhesive, and plastic additive, and is approved US FDA indirect food additive. TMDD occurrence is attributed to its use in printing inks, paints, and recycled paper, and was documented in wastewater and river water in Germany (19), but no reports in foods were found. 2,2,4-trimethyl-1,3-pentanediol diisobutyrate (TXIB) is a low-viscosity plasticizer, and is also approved by the US FDA as an indirect additive. Reports on TXIB migration from plastic baby bottles were previously published (9,11).
Linear Alkylbenzenes
Six linear alkylbenzenes (LABs) (Table II, #60-65) were identified in the extracts. LABs are used in the manufacture of linear alkylbenzene sulfonates used as surfactants for household detergents, and are considered an indicator of human activities associated with sewage contamination. LABs have high octanol water partition coefficients (7–10), and have been reported in environmental samples (20).
PCBs
The discovery of the five PCB congeners (Figure 2) was unexpected. The identified PCBs contain two or three chlorines. Typical PCB profiles in environmental and biological matrices (for example, sediment or tissue), contain 30 to 50 congeners with a greater range of chlorination. PCBs were banned in the US in 1979 due to their carcinogenicity in humans and animals (21). However, due to their persistence, PCBs are still ubiquitous in the environment. Human health risk due to PCB exposure is actively monitored, for example, through sportfish consumption surveys. We suspect the PCBs identified in this experiment are by-products of the film manufacturing process. A recent study found that PCBs with one or two chlorines were detected at most abundance among the 50 PCB congeners in paint pigments (22), which are commonly used in several products, including plastics. The discovery of PCBs in the film extracts suggests their source should be investigated further.
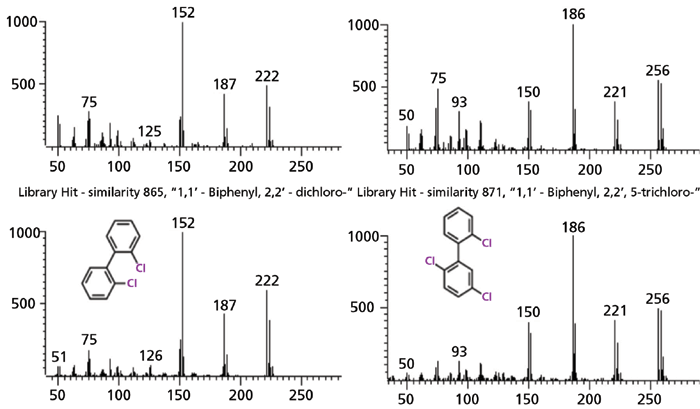
Figure 2: Mass spectra identified as polychlorinated biphenyls (PCBs): the left shows a 86.5% match with 2,2'-dichlorobiphenyl and the right shows a 87.1% match with 2,2',5-trichlorobiphenyl from the US National Institute of Standards and Technology (NIST) library. The right spectrum was further confirmed by match to an authentic standard, 2,2',5-trichlorobiphenyl. For all figures shown, the x-axis is mass (m/z) and the y-axis is relative abundance.
Miscellaneous Others
We think that identified compounds 10,18-bisnorabieta-8,11,13-triene and 13-isopropylpodocarpa-8,11,13-trien-19-al, a derivative of PAH phenanthrene, could be thermal degradation products based on their structural similarity to 1-methyl-10,18-bisnorabieta-8,11,13-triene, which was identified as an intermediate compound formed during thermal degradation of adhesives in food packaging (5). Additionally, phenyl/biphenyl/diphenyl compounds were identified in the extracts.
Ground Beef Exposure
While the data analysis on identification of chemicals migrated from plastic film into beef samples is underway, we identified 182 and 102 chromatographic peaks with area three times larger than in control in defrost and microwave exposed samples, respectively. Some of the tentatively identified chemicals included benzophenone (Figure 3) and 2,4,7,9-tetramethyl-5-decyne-4,7-diol, which were previously found in the film extracts. The latter is an approved FDA indirect additive. Interestingly, two compounds were tentatively identified in exposed beef samples, which were not found in the film extracts: 1,2,3,4-tetrahydro-1,1,6-trimethylnaphthalene, used in manufacture of polymers, and 1-phenyl-5-methylheptane, a precursor of biodegradable detergents. Possibly, introduction of two additional variables: microwave heat and beef fat content are responsible for this finding. Similarly, it was reported that migration of Irganox 1076, an antioxidant in food packaging polymers, had increased from plastics into foods and food simulants with increased temperature and fat content (23). This finding suggests the importance of studying migration into real food samples when possible, under controlled conditions.
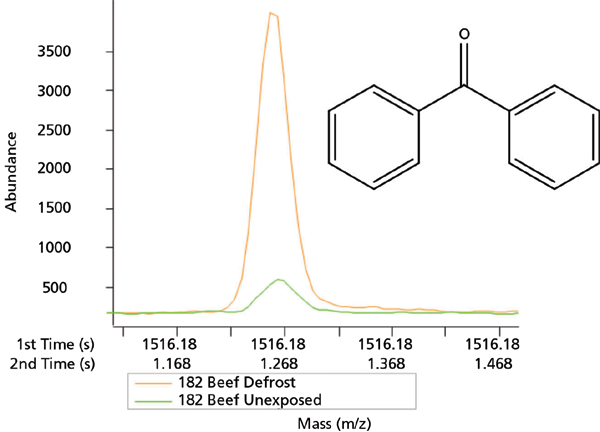
Figure 3: Benzophenone identified in the ground beef exposed to plastic film during microwave defrosting (red line) versus control (unexposed) ground beef (green line) sample. The x-axis represents GC retention times and y-axis represents abundance of m/z 182.
Conclusions
In this study, we tentatively identified and characterized chemicals extracted from food packaging plastic stretch film into FDA food simulant and organic solvents: hexane, ethyl acetate and acetonitrile. GC×GC–TOF-MS analysis based on ≥70% match similarity to the standard NIST mass spectral library and manual review of mass spectral matching was used for identification. Comparison of different solvents for extraction resulted in 91 identified compounds in ethyl acetate, 74 in acetonitrile and hexane, and 64 in ethanol–water extracts, with some compounds showing selective migration into some solvents versus others. Overall, several classes of chemicals were identified among the extracted compounds: alkylated naphthalenes used as lubricants; polycyclic aromatic hydrocarbons (PAHs) including those on the US EPA priority pollutant list; common used plasticizers, polymer additives, UV filters, flavoring agents and fragrances, surfactants, adhesives, products of thermal degradation, and low chlorinated PCBs. Several compounds tentatively identified in our study were not previously reported as migrants from plastic materials by previous studies, which may be attributed to the specific plastic film studied. Future studies should focus on quantitative migration to estimate the amounts of migrating chemicals into food simulants and real foods to provide data for risk assessment.
References
(1) L.W. McKeen, in Plastic Films in Food Packaging, S. Ebnesajjad , Ed. (William Andrew Publishing, Oxford, 2013), pp. 1–15.
(2) B. Li, Z.W. Wang, Q.B. Lin, C.Y. Hu, Q.Z. Su, Y.M. Wu, J. Chromatogr. Sci.53, 026–1035 (2015).
(3) M.A. Lago and L.K. Ackerman, Food Addit. Contam. A33, 518–529 (2016).
(4) A. Vavrouš, L. Vápenka, J. Sosnovcová, K. Kejlová, K. Vrbík, and D. Jírová, Food Control 60, 221–229 (2016).
(5) C. Nerin, P. Alfaro, M. Aznar, and C. Domeno, Anal. Chim. Acta. 775, 14–24 (2013).
(6) C. Bignardi, A. Cavazza, C. Corradini, and P. Salvadeo, J. Chromatogr. A1372, 133–144 (2014).
(7) M.J. Martinez-Bueno, M.D. Hernando, S. Ucles, L. Rajski, S. Cimmino, and A.R. Fernandez-Alba, Talanta172, 68–77 (2017).
(8) M. Bonini, E. Errani, G. Zerbinati, E. Ferri, and S. Girotti, Microchem. J. 90, 31–36 (2008).
(9) M. Onghena, E. van Hoeck, P. Vervliet, M.L. Scippo, C. Simon, J. van Loco, and A. Covaci, Food Addit. Contam. A31, 2090–2102 (2014).
(10) M. Boccacci Mariani, E. Chiacchierini, and C. Gesumundo, Food Addit. Contam.16, 207–213 (1999).
(11) C. Simoneau, L. Van den Eede, and S. Valzacchi, Food Addit. Contam. A29, 469–480 (2012).
(12) Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on Polycyclic Aromatic Hydrocarbons in Food, EFSA J. 724, 1–114 (2008).
(13) O.W. Lau and S.K. Wong, J. Chromatogr. A882, 255–270 (2000).
(14) I.S. Arvanitoyannis, and L. Bosnea, Crit. Rev. Food Sci.44, 63-76 (2004).
(15) United States Food and Drug Administration. Indirect additives used in food contact substances. https://www.accessdata.fda.gov/scripts/fdcc/?set=IndirectAdditives. Accessed May 11, 2018.
(16) X.L. Cao, W. Zhao, R. Churchill, and C. Hilts, J. Food Protect.77, 610–620 (2014).
(17) A.E. Goulas, K.I. Anifantaki, D.G. Kolioulis, and M.G. Kontominas, J. Dairy Sci. 83, 1712-1718 (2000).
(18) M. Strangl, T. Fell, M. Schlummer, A. Maeurer, and A. Buettner, J. Sep. Sci.40, 1500-1507 (2017).
(19) A.A. Guedez, and W. Püttmann, Sci. Total Environ. 468–469, 671–676 (2014).
(20) K.O. Isobe, M.P. Zakaria, N.H. Chiem, L.Y. Minh, M. Prudente, R. Boonyatumanond, M. Saha, S. Sarkar, and H. Takada, Water Research38, 2449–2459 (2004).
(21) Health effects of PCBs. U.S. Environmental Protection Agency. 13 June 2013. https://en.wikipedia.org/wiki/Polychlorinated_biphenyl.
(22) D.F. Hu and K.C. Hornbuckle, Environ. Sci. Technol.44, 2822–2827 (2010).
(23) G. Beldi, S. Pastorelli, F. Franchini, and C. Simoneau, Food Addit. Contam. A 29, 836–845 (2012).
Yelena Sapozhnikova is with the Agricultural Research Service of the USDA, in Wyndmoor, Pennsylvania. Direct correspondence to: yelena.sapozhnikova@usda.gov
Eunha Hoh is with the School of Public Health at San Diego State University.

Analysis of PFAS in Milk by LC-MS/MS
May 15th 2025Dairy milk is one commodity that can be impacted by environmental contaminants, such as PFAS, so it is important to implement extensive, robust, and accurate testing. In this work, a sensitive and reliable method was developed for the analysis of PFAS in milk by LC-MS/MS at levels as low as 0.01 µg/kg.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











