Supporting Bioanalysis with Dried Blood Spots
LCGC Europe
Blood is perhaps the most widely used sample fluid in bioanalysis. Dried blood spot (DBS) sampling has been used in clinical applications for more than 50 years, but it is recently seeing a resurgence of interest. DBS sampling holds several advantages associated with the use of small sample sizes obtained via finger pricks, including simplicity and biohazard reduction. In the previous instalment, we gave an overview of microsampling in bioanalysis (1). This month, we dig deeper into bioanalysis using DBS sampling.
Douglas E. Raynie, Sample Preparation Perspectives Editor
Blood is perhaps the most widely used sample fluid in bioanalysis. Dried blood spot (DBS) sampling has been used in clinical applications for more than 50 years, but it is recently seeing a resurgence of interest. DBS sampling holds several advantages associated with the use of small sample sizes obtained via finger pricks, including simplicity and biohazard reduction. In the previous instalment, we gave an overview of microsampling in bioanalysis (1). This month, we dig deeper into bioanalysis using DBS sampling.
Bioanalysis has been the trendy topic in analytical chemistry recently because of advances in the “omics” fields and the development of increasingly sensitive instrumentation and detection. In a “what goes around comes around” situation, dried blood spot (DBS) sampling is seeing a resurgence for use with biological fluids. Grüner, Stambouli, and Ross (2) traced the use of DBS in the determination of glucose and Kjeldahl nitrogen more than 100 years ago and later for diagnosis of certain disease states. Most recently, the field of DBS sampling has grown significantly. Figure 1 shows the number of peer-reviewed publications that used the keyword “dried blood spot” during the past 15 years. In particular, during the last 7–9 years growth in the reports of DBS sampling has increased rapidly.
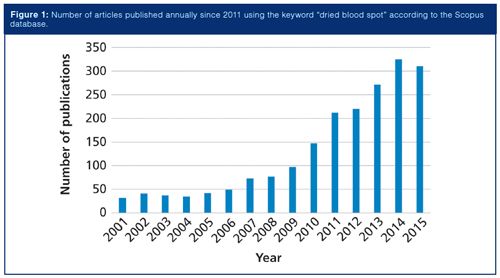
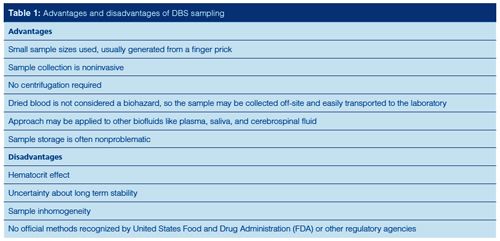
The resurgence of interest in DBS sampling for bioanalysis stems from the several advantages it offers. Blood is the most frequently sampled biofluid and the collection of a droplet onto a DBS card is quite simple. After the sample dries, it is generally considered not to be a biohazard. Thus, samples could potentially be collected at home by patients or clinical trial participants and mailed to clinical laboratories. Perhaps the largest drawback to DBS sampling is the so-called hematocrit effect, the ratio of red blood cells to plasma, which can impact sample viscosity and homogeneity. The impact of this effect is strong enough to warrant a separate discussion.
DBS Sampling and Analysis
Sample collection in the DBS approach seems rather straightforward, but there are several important considerations. It is recommended that the second drop be collected, rather than the initial drop to avoid bias from increased levels of tissue fluid. Usually specially designed filter papers, or “cards”, are used for DBS analysis, and care must be taken to avoid smearing the blood on the card during collection (3). The DBS card must be handled carefully to avoid contamination and the following procedure is typical in DBS sampling (4). After the skin is cleaned in the area where the sample is to be drawn, a lancet may be used to pierce the skin and the first blood drop is wiped away with a cotton ball. Up to five drops (one drop equals about 10 µL) are allowed to fall onto the DBS card. To avoid smearing, as previously described, the finger should not touch the card during sampling. A few hours, with exposure to air on both sides of the collection matrix, are needed to adequately dry the blood samples. When returning the sample to the laboratory, the cards are often placed in plastic bags, with or without desiccant, and stored at room temperature, or refrigerated or frozen.
Method validation with DBS analysis varies since it appears that sample stability may change with storage conditions, including time and temperature. Internal standards are often added to the blood or card before or after sampling. One of the previously stated disadvantages of DBS analysis was sample inhomogeneity. This may be caused by the hematocrit effect, filter paper (card) consistency, humidity, and drying conditions.
After the DBS card is brought to the laboratory, the card may be analyzed as is or a precisely measured disk is punched from the blood spot. The blood spot may be extracted with solvent, purified via solid-phase extraction, and injected directly into a chromatographic system with or without derivatization. In a cursory review of the literature over the past three years, it appears that liquid chromatography–tandem mass spectrometry (LC–MS/MS) is becoming the most common analytical tool for DBS sampling. In any case, the analytical procedure must be highly sensitive because of the small sample volumes, plus it should be tolerant of proteins and other potential interferences that may not be removed during the work-up.
The DBS cards are typically made from cellulose, carboxymethyl cellulose, chemically treated cellulose, chitosan, or alginate, with cellulose being the most popular. Differences in DBS cards, even between vendors, are because of composition and thickness of the absorbent material.
Based on these analytical considerations, DBS sampling has been used in myriad applications, including therapeutic drug monitoring, clinical trials, cancer therapy and immunosuppressive investigations, pollutant analysis including triazole antifungals, pharmacokinetics, diagnosis and mapping of diseases, doping tests in sport, animal sampling for research purposes, neonatal metabolic studies, and drug metabolism evaluation. Although DBS sampling is not recognized by regulatory agencies, the European Bioanalysis Forum established good bloodâspotting practices (5,6).
Effect of Hematocrit
The single largest limitation to DBS sampling is the hematocrit effect. While the hematocrit effect is still under study, the impact on DBS analysis is likely because of differences in viscosity. Blood viscosity will dictate the amount of sample in a spot and the red blood cell-to-plasma ratio is directly related to the analyte concentration. DBS size decreases with increasing blood viscosity. High hematocrit ratios can result in decreased analyte concentrations with extracted samples. Wilhelm and colleagues (7) showed that as hematocrit levels rose, the blood spot did not spread as well on the cellulosic filter paper, was less homogeneous, and was more concentrated. Although the impact of hematocrit is well established, how to control the effects of the variability resulting from this phenomenon is not. This variability remains the biggest challenge to more wide spread adoption of DBS sampling.
Conclusions
Because of the simplicity of sampling and other advantages, the DBS approach shows great promise in the bioanalytical world. Although inconsistencies resulting from hematocrit effects must be confronted and regulatory acceptance is lacking, additional research will answer these deficiencies, rendering DBS analysis commonplace for coming generations.
References
- D.E. Raynie, LCGC Europe29(8), 442–445 (2016).
- N. Gruner, O. Stambouli, and R.S. Ross, J. Visual. Exper. 97, 1–9 (2015).
- D. McMorran, D.C.K. Chung, M. Toth, O.W. Liew, M. Muradoglu, and T.W. Ng, Anal. Biochem.506, 28–30 (2016).
- M.V. Antunes, M.F. Charão, and R. Linden, Clin. Biochem. 49, 1035–1046 (2016).
- M. Wagner, D. Tonoli, E. Varesio, and G. Hopfgartner, Mass Spectrom. Rev.35, 1–78 (2016).
- P. Timmerman, S. White, S. Globig, S. Lüdtke, L. Brunet, and J. Smeraglia, Bioanalysis3, 1567–1575 (2011).
- J.A. Wilhelm, J.C.G. den Burger, and E.L. Swart, Clin. Pharmacokinet. 53, 961–973 (2014).
“Sample Prep Perspectives” editor Douglas E. Raynie is an Associate Research Professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high resolution chromatography, and bioprocessing in supercritical fluids. He earned his Ph.D. in 1990 at Brigham Young University under the direction of Milton L. Lee.

New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










