Stability of Formic Acid in Methanol Solutions and the Implications for Use in LC–MS Gradient Elution Analysis
LCGC North America
Dilute formic acid solutions in methanol were found to decline in acid content with time, the extent of the decline depending upon the initial amount of water present in these solutions. The effect of the formic acid concentration change upon the separation of peptides using high performance liquid chromatography (HPLC) is examined briefly.
Solvent blends modified with small amounts of additives are extensively used for performing gradient high performance liquid chromatography (HPLC) analyses (1). Many solvent–water–additive combinations are employed. Acidic modifiers are used extensively for the analysis of proteins of various molecular weights. In the case of HPLC coupled with mass spectrometry (MS) detection, acid additives (or modifiers) such as trifluoroacetic acid, formic acid, and acetic acid often are used (2).

Formic acid as a solvent modifier has been used extensively as an alternative to trifluoroacetic acid. This is due to formic acid's relatively lower ability to suppress fragment ion detection in time of flight (TOF)–MS analysis using electrospray ionization (ESI) of samples compared with trifluoroacetic acid (3). While there is less signal suppression with formic acid compared with trifluoroacetic acid, there is a possible sacrifice of signal resolution with a formic acid modifier.
In earlier work, we showed that the concentration of formic acid in acetonitrile and water gradient solutions for LC–MS analysis of certain peptides critically affected the success of the analysis (4). This finding indicated that careful control of the formic acid content of solvent blends used for gradient chromatography was important for success.
In our work examining the properties of formic acid–modified solvent and water blends, we observed that some of these blends were not stable with time when stored at ambient temperatures. In particular, we noted that 0.1% (v/v) formic acid blends in methanol significantly and often rapidly degraded with time. We detected these changes by titrimetrically monitoring the free acid content over time of freshly prepared samples. This note summarizes our findings concerning the stability of formic acid–methanol blends.
Experimental
GC–MS data were collected using Shimadzu GCMS-QP2010 and GC-2010 systems (Columbia, Maryland) with a Supelco 105m RTX-1 column (Bellefonte, Pennsylvania). The column flow was 2 mL/min, and the split ratio was 5:1. The temperature program was 10 min at 60 °C, 5 °C/min to 260 °C, hold 12 min. The scan range was 15 to 450 m/z, and the EI voltage was 70 kV.
LC–MS data were collected using an Agilent 1200 Series HPLC system (Santa Clara, California) and a Gemini Phenomenex C18 column (150 mm × 2.0 mm, 3 μm particles) (Torrance, California) with a TOF–MS detector. UV detection at 245 nm also was employed. Gradient method: At 0 min, 95% A (0.1% formic acid–water); at 50 min, 30% B (0.1% formic acid–methanol); at 80 min, 60% B; at 85 min, 5% B hold until 87 min. The flow rate was 0.20 mL/min. The column was thermostated at 30 °C. MS–TOF conditions: ion polarity was positive; m/z range was 100–2800; the gas temperature was 350 °C; the ESI capillary voltage was 3 kV; and the fragmenter voltage was 180 V.
Titration data were collected over time using a Metrohm 736 GP Titrino autotitrator (Houston, Texas). A dilution of three parts water to one part formic acid blend was used to prepare the titration samples. The titrant was a 0.1 N potassium hydroxide solution in methanol.
Fluka Chemika formic acid (Buchs, Switzerland) and Honeywell Burdick & Jackson B&J Brand methanol and water (Muskegon, Michigan) were used to prepare the formic acid in methanol and the formic acid in methanol–water blends. All methanol and water solutions were prepared by volume. Formic acid was then added by volume to prepare the various 0.1% (v/v) formic acid in methanol–water blends that were evaluated. The solutions were kept in sealed bottles and at ambient temperature, 20–22 °C.
A nine-peptide test mixture from Sigma was used for HPLC performance evaluations (bradykinin, bradykinin fragment 1-5, substance P, [Arg8] — vasopressin, Luteinizing hormone releasing hormone, bombesin, leucine enkephalin, methionine enkephalin, and oxytocin).
Results and Discussion
When 0.1% (v/v) formic acid in methanol blends were prepared in the laboratory or on a large scale, we noted that the acid content of such blends appeared to change rapidly with time at ambient temperature. Figure 1 shows the results of titrimetrically determined acidity of such blends. We can estimate the first half-life of this particular blend to be approximately 88 h.
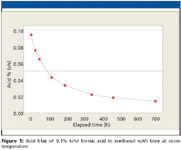
Figure 1
We subsequently examined the blend using GC–MS (Figure 2). The peak at 10.6 min was identified as methyl formate, the esterification reaction product of formic acid and methanol. That this ester formed so rapidly in an anhydrous system at room temperature was not surprising (5,6).
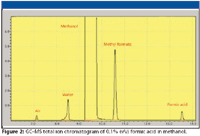
Figure 2
We decided to determine whether or not this reaction might still occur if water was added to the formic acid–methanol blend. It was anticipated that increasing the amount of water in the originally anhydrous blend would slow the reaction down sufficiently to provide a longer first half-life for the blend. To this end several 0.1% (v/v) formic acid in methanol–water blends were prepared and the acid value monitored with time. The collated data appear in Figure 3.
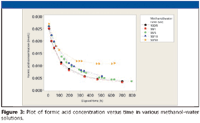
Figure 3
It is clear from the data that even the water content of a 50:50 (v/v) methanol–water blend did not slow down appreciably the initial rapid loss of formic acid. The blend is more stable at higher water contents: after approximately 2400 h, the acid titer for a 95% water blend was 0.09%. The 100% water blend is stable with time. Table I shows the first half lives estimated from the plots of Figure 3. Please note that the samples were kept at ambient temperature, and not in a thermostat at a constant temperature.
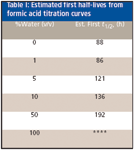
Table I: Estimated first half-lives from formic acid titration curves
With our available data, we used the method of characteristic kinetic plots to attempt to determine the reaction order of the esterification reaction (7). Figures 3–5 show the characteristic plots obtained. Examination of these plots suggest that the solutions of 0.1% (v/v) formic acid in methanol with 0, 1, 5, and 10% (v/v) water content appear to follow a second order rate law (Figure 5). This was somewhat unexpected, since a pseudo-first order reaction rate was anticipated [large excess of one reactant (methanol) reacting with a limited amount of a second reactant (formic acid].
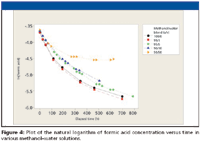
Figure 4
We further evaluated the data using Minitab 14 software (State College, Pennsylvania). Regression analysis of the data used for Figure 5 indicated that there was possible curvature to the plots of data for 0, 1, 10, and 50% water content, with no apparent curvature detected for 5% water content. This suggests that the overall mechanism can be more complicated than what we have interpreted from the data (8).
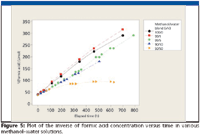
Figure 5
From the regression analysis of the data shown in Figure 5, we estimated the putative second order rate constants for the 0, 1, 5, and 10% water content blends. These are shown in Table II. The substantially faster p-toluenesulfonic acid catalyzed esterification rate for anhydrous formic acid in methanol at 30 °C is 0.308 L/mol-s (9).

Table II: Estimated second order rate constant from characteristic kinetic curve regression analysis
Given the observed substantial loss of formic acid content over relatively short time periods, we investigated how this change in formic acid concentration might affect HPLC performance. To accomplish this, we recorded several HPLC gradient chromatograms using water and methanol, each solvent being modified with an initial concentration of 0.1% (v/v) formic acid.
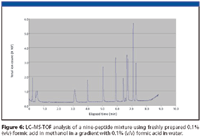
Figure 6
Figure 6 shows the gradient separation of the nine-peptide mixture using a freshly prepared solution of 0.1% (v/v) formic acid in methanol. Figure 7 shows the gradient separation observed using the same solution of 0.1% (v/v) formic acid in methanol after two days aging. A slight shift in retention times has occurred, with some change in resolution.
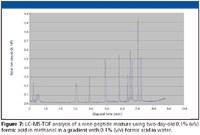
Figure 7
The apparent small effect of formic acid concentration in methanol upon the chromatography of the nine-peptide solution led us to explore whether or not formic acid in methanol was required at all for a successful separation of this nine-peptide mixture. The chromatogram of Figure 8 shows that some amount of formic acid in methanol is required for baseline stability and for better chromatography for this nine-peptide mixture.
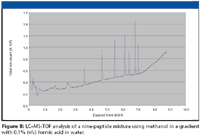
Figure 8
Conclusion
Blends of formic acid in methanol with and without added water are not stable and can affect adversely some types of chromatographic applications.
Karel A.J. Snoble, Neal M. Fox, Sandra M. Lorenz, Anthony R. Kemperman, and J.T. Przybytek
Honeywell Burdick & Jackson Muskegon, Michigan
Please direct correspondence to Karel A.J. Snoble at karel.snoble@honeywell.com
References
(1) Snyder and Kirkland, Introduction to Modern Liquid Chromatography, Second Edition (John Wiley & Sons, Inc, New York, 1979).
(2) M.C. Garcia, J. Chrom., B 825(2), 111–123 (2005).
(3) C.G. Huber and A. Premstaller, J. Chrom., A 849, 161–173 (1999).
(4) A. Kemperman, P. Scheidle, J. T. Przybytek, K. A. Snoble, K. D. Ulrich, and C. L. Seaver, Design of High Purity Blends for Chromatography Utilizing Six Sigma Methodology: Part 2 – Commercialization, Poster #150-1 presented at PITTCON 2006.
(5) R. Boekmeier, Praxis der Naturwissenschaften, Chemie 36(5), 7–10 (1987).
(6) A.E. Sommerfield, School Science Review, 54(189), 769-70 (1973).
(7) See www.chm.davidson.edu/ChemistryApplets/index.html for a discussion of the topic. See also M. R. Wright, An Introduction to Chemical Kinetics (John Wiley & Sons Ltd., West Sussex, UK, 2004).
(8) L.F. Fieser and M. Fieser, Advanced Organic Chemistry (Reinhold Publishing Corporation, New York, 1961), pp. 372–373.
(9) K.E. Schulte and J. Kirschner, Fette und Seifen 53, 267–271 (1951).

Determining the Effects of ‘Quantitative Marinating’ on Crayfish Meat with HS-GC-IMS
April 30th 2025A novel method called quantitative marinating (QM) was developed to reduce industrial waste during the processing of crayfish meat, with the taste, flavor, and aroma of crayfish meat processed by various techniques investigated. Headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) was used to determine volatile compounds of meat examined.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











