Split, Splitless, and Beyond—Getting the Most From Your Inlet
LCGC Europe
While capillary gas chromatography has been undergoing a renaissance, with new columns, detectors, data systems, and multidimensional separations, the classical inlets have remained the same: We are still injecting liquid samples with syringes into split and splitless inlets, as we have for nearly 50 years. Split and splitless injections present several well-known and some not-so-well known challenges, mostly arising from heating of the inlet, that make sample injection and inlets a major hurdle for gas chromatographers. These challenges and some ideas for mitigating them are discussed and a case is made for renewed exploration of the cool inlets and injection techniques: cool on-column and programmed temperature vaporization.
While capillary gas chromatography has been undergoing a renaissance, with new columns, detectors, data systems, and multidimensional separations, the classical inlets have remained the same: We are still injecting liquid samples with syringes into split and splitless inlets, as we have for nearly 50 years. Split and splitless injections present several well-known and some not-so-well known challenges, mostly arising from heating of the inlet, that make sample injection and inlets a major hurdle for gas chromatographers. These challenges and some ideas for mitigating them are discussed and a case is made for renewed exploration of the cool inlets and injection techniques: cool on-column and programmed temperature vaporization.
Gas chromatography (GC) has seen some tremendous advances over the past two decades. New instruments have come to take much fuller advantage of the capabilities of capillary columns. New technologies include sorptive microextraction techniques such as solid-phase microextraction (SPME), fast GC, comprehensive multidimensional GC (GC×GC), capillary columns that are highly inert (mass spectrometry [MS] designated) and selective (specialty and ionic liquid), and new detectors, including benchtop MS-MS and vacuum ultraviolet (VUV) detectors. They demonstrate that new research on GC is still coming. It is poised to remain a staple analytical technique well into the future.
With all of that development, it is ironic that the one area that perhaps baffles chromatographers the most, the inlet, has received little attention. Four inlets are used on main-line instruments: split, splitless, on-column, and programmed temperature vaporization (PTV). By far the most commonly used inlets are split and splitless, which were developed in the 1950s and 1960s; the most recently developed inlet, PTV, was invented in 1979. Although computer control of pneumatics and highly inert glass sleeve materials have marginally improved split and splitless as inlets, the fundamental problems that have baffled chromatographers for 50 or more years remain.
Split and Splitless-The Old Reliable Standbys
The vast majority of gas chromatographs manufactured today include a single inlet, often called a split–splitless inlet, that performs two types of liquid sample introduction: split and splitless. To varying degrees, split and splitless injections address five fundamental problems with injecting liquid samples into a capillary GC system:
- The needle problem: Most syringe needles do not fit into a capillary column.
- The mass problem: You can only inject small amounts of sample onto a capillary column (1 µL of a liquid sample weighs about 1 mg) and you never know how much sample you actually injected.
- The time problem: You never know exactly how long the injection process takes or how wide the peaks will be at the head of the column.
- The contamination problem: Dirty samples are bad for the column, and contaminants can collect in the inlet or at the column head and interact or react with analytes. Analytes can also interact with the surfaces inside the inlet.
- The discrimination problem: Heating of the inlet or syringe needle can cause some analytes to remain in the needle or inlet while others transfer to the column.
A schematic diagram of a split–splitless inlet is shown in Figure 1. Note that the inlet has a high thermal mass and is typically heated to a temperature well above the normal boiling point of most samples, often 250 °C. As seen in the figure, the five fundamental problems are partially addressed by the inlet design and how it is operated. A simple review of some basic principles can be accessed online at LCGC Europe’s ChromAcademy (1). For a discussion of the basics of split and splitless inlet maintenance, see the May 2018 issue of LCGC Europe (2). Grob’s classic book on split and splitless injection provides over 800 pages of fundamentals, theory, and practice related to split and splitless injections (3).
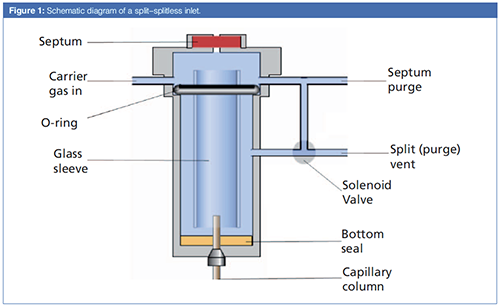
The needle problem is addressed by a glass sleeve, which serves as a venue for the transfer of the sample from the syringe needle to the column. All common syringe needles can easily fit into the glass sleeves used in split and splitless inlets. The mass problem is clearly evident in a split injection, as can be illustrated with a simple example. A 1-mg (1-µL) amount of a liquid sample with a 100:1 split ratio allows about 10 µg of sample to reach the column. If the analyte concentration is 1 ppm, then the mass reaching the detector, assuming none is lost in the column or inlet, can be estimated as 10 pg, which is below the detection limits of many detectors. Split injection, although considered simple, is very limited for trace analysis.
Splitless injection partially mitigates this problem because it allows introduction of nearly all of the injected sample into the column. In the above example, the split ratio would not be included, so the mass going onto the column is estimated as 1 ng, which is much more suitable for most detectors. A second mass problem arises with the term “estimated” to describe the mass of analyte reaching the column in both of the above examples. Although calibration techniques can mostly mitigate this problem for most quantitative analysis, the actual mass of analyte reaching the column is not accurately known in either split or splitless injection.
Recently, Bai and colleagues used vacuum ultraviolet detection, which allows for pseudo-absolute quantitation without using standards, to examine the efficiency of split and splitless injections (4). They analyzed several variables, including split ratio, splitless “purge off” time, and injection volume. In an investigation of split ratio, in all cases with split ratios ranging from 5:1 to 200:1, the actual mass measured by GC–VUV was significantly lower than the expected mass calculated using the sample concentration, injection volume, and split ratio. Performance was seen to get worse at lower split ratios.
In both split and splitless injection, it is important to avoid overloading the glass sleeve with too much vapour when the sample evaporates. Many typical glass sleeves have a volume of 1 mL or less, but depending on the solvent, a 1-µL liquid volume can have a volume of 200 µL to 1.2 mL or more when it is fully evaporated. To assist in addressing this problem, there are several solvent vapour volume calculators available online (1,5–6). These calculators are useful for estimating the solvent vapour produced during the injection process and comparing it to the volume of typical glass sleeves. Be sure to keep the solvent-vapour volume lower than the glassâsleeve volume. This effect can also contribute to mass problems if solvent vapour backflushes into the gas lines that feed the inlet, taking some of the vaporized analytes with it.
In splitless injections and to a small extent in split, especially at low split ratios, the time problem presents a significant challenge, termed by Grob (3) as “band broadening in time”. During the “purge off” time period in a splitless injection, gas flow through the glass sleeve is reduced to match flow in the column. For many typical capillary columns, this flow rate is about 1 mL/min. If the glass sleeve has a volume of about 1 mL then it can take about 1 min for the carrier gas to sweep the injected sample into the column, so the initial peak reaching the column is about 1-min wide!
Figure 2 shows some profiles from actual peaks as they leave the inlet under splitless conditions (7,8). These profiles were obtained by placing a very short empty fusedâsilica transfer line between the inlet and a flame ionization detector, and using SPME to perform the injection (so there is no solvent present). Note that the peaks are about 1-min wide and are not fully symmetrical. The bulk of the peak broadening is due to the time required for the sample to traverse from the SPME fibre through the glass sleeve and into the column. Note also that traces of the sample may remain in the inlet for a long period of time, often much longer than the “purge off” time. Minimizing the time required for the sample to traverse the glass sleeve should be a method development goal.
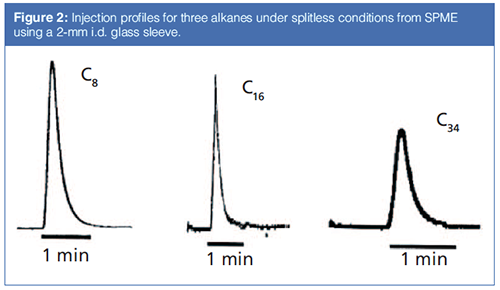
Clearly, the peaks resulting from most splitless injections do not end up 1 min wide at the detector and on chromatograms. There are several peak-focusing mechanisms at play, all described in great detail in Grob’s book (3). These mechanisms lead to some important optimization points specific to splitless injections:
- Use cold trapping to refocus highâboiling solutes. If possible, start your temperature program at least 100 °C below the normal boiling point of the analytes.
- Use solvent effects to refocus low-boiling solutes. Start your temperature program 30–40 °C below the normal boiling point of the solvent. For most solvents and for convenience I start it at 40 °C. The impact of solvent effects decreases as the analyte normal boiling point increases.
- Use a narrow-bore straightâtube glass sleeve, but beware of possible liner overload as the solvent evaporates. Obstructions and materials such as glass wool collect dirt and can cause as many problems as they solve.
- Match the polarity of the solvent to the polarity of the column. Use nonpolar solvents with nonpolar columns.
To take advantage of these optimizations, all methods using splitless injection should be temperature programmed.
Contaminant problems relate to the composition of the sample, and the chemistry of the surfaces inside the inlet and column can be especially challenging. Obviously, dirty samples can shorten column life by fouling the column or the inlet. The design of split–splitless inlets that use a glass sleeve does prevent some column fouling by providing a landing place for nonvolatile sample components. However, it is possible for nonvolatile sample components or residual components left behind after splitless injections to remain on the surfaces and contaminate them.
Thinking about samples interacting with the surfaces within the inlet leads to a simple question with a surprisingly complicated answer.
What Really Happens When a Liquid Sample Ejects From a Syringe Into a Split–Splitless Inlet?
Most short courses and books introducing GC tell us that the injected sample is flash vaporized and then mixed with the carrier gas and transferred as a homogenous mixture in the vapour phase into the column. Numerous glass sleeves have been designed to facilitate this concept. However, two common sense experiments demonstrate that this description of the process is not accurate and that care is required to avoid reactions and contamination in the inlet.
Figure 3 shows the result when a small volume of about 5 mL of water-analogous to the sample-is poured onto a hot cast iron skillet, analogous to the hot inlet surfaces. This experiment was done at home, but it also works in the laboratory with a hot plate. Not only does the water not evaporate immediately, but, because of the formation of a vapour layer between the water and the surface, it is seen to dance. This is what really happens when a liquid sample strikes the surfaces within a heated inlet. It is clear that the evaporation processes in heated inlets are not easily controlled and certainly are not reproducible. This effect is also the primary cause of syringe needle discrimination, which is common in manual injections and is caused by the heating of the syringe needle in the inlet.

The second simple experiment is to simply see how far you can shoot some water with a typical 10-µL syringe, used for GC. It is easy to shoot the liquid much farther than the length of the inlet. This experiment demonstrates that the liquid leaving the syringe is not likely to spread out and flash vaporize upon exiting the syringe. It is likely to shoot straight to the first surface it encounters, then evaporate in a manner analogous to the skillet example. Grob’s book (3) includes a DVD with several videos demonstrating this process. A few of these videos can be seen online (9).
These heating effects are the primary cause of the fifth challenge: discrimination. They can cause some analytes to preferentially evaporate and be carried to the column, whereas other components do not evaporate as efficiently and are only partially transferred or do not enter the column at all. Because the heating is not controlled well, neither is the discrimination. Discrimination and contamination in the inlet are leading causes of precision and accuracy problems in gas chromatography.
Based on the five challenges, the following quick-hitting keys can help analysts reduce discrimination and contamination and get the most from classical split and splitless inlets:
- Keep the inlet heated and with carrier gas flow at all times to reduce contamination. Gas saver mode between runs will reduce carrier gas consumption.
- Use a fast autosampler for liquid sample injections-faster is better.
- Use the best glass sleeve for your sample. The sleeves for split and splitless are different. There is no magic bullet sleeve for all samples. You should test several different sleeves when optimizing your method.
- Check cleanliness and clean the inlet on a regular basis. Use a flashlight to look for debris at the bottom of the inlet, and clean it out if you see any.
- Change the septum often. The wide, blunt needles used with many autosamplers can core them quickly and cause leaks.
Why Are We Still Doing Hot Injections?
Most of the challenges involved with using split and splitless inlets occur because the inlet is heated. The reasons for heating these inlets arose from packed-column GC, in which strong heating is the only way to make analytes pass through the column. In classical packed columns, the large mass of stationary phase present in the column in gas–liquid chromatography, or a large surface area in the case of gas–solid chromatography, generally means that analyses are carried out at temperatures above the normal boiling points of the analytes. Strong heating throughout the separation, including injection, separation, and detection, is therefore required. Packed-column inlets are also generally very simple: The syringe needle fits directly into the column, so many of the problems with capillary inlets described above do not occur.
In capillary GC, the low mass of stationary phase present in the column changes the equilibrium conditions on which GC separations are based. With most thin and moderate film thicknesses, separations are conducted at temperatures below the normal boiling points of the analytes. Classical split and splitless injections therefore involve ejection of the sample from the syringe, evaporation in the glass sleeve, transfer of the vapour through the glass sleeve into the column, and condensation into the stationary phase in the column. Each of these steps may involve complex chemical interactions and, as shown in Figure 3, they may occur in an uncontrolled fashion. This possible problem leads to the question, “Why are we still doing hot injections?”
A false answer to that question is that there are no alternatives. There are two alternatives available for nearly every capillary gas chromatograph: on-column inlets and PTV. Both of these inlets eliminate most of the contamination and discrimination problems related to the hot injection and surface of the glass sleeve by injecting the sample into a cool inlet instead of a hot inlet. This approach keeps both the syringe needle and inlet cool throughout the injection process. The inlet is then heated to move analytes into the column after the syringe is removed. On-column and PTV inlets have been available for decades, yet they remain niche techniques even though they can reduce or eliminate the major complaints about split and splitless inlets.
An on-column inlet is exactly that: The syringe needle is inserted directly into the end of the column and the liquid sample is deposited onto the column head. The inlet is then temperature programmed along with the column to generate the separation. Using this inlet completely eliminates heating during the injection and the glass sleeve, removing all of the problems described above that these steps can generate. Cool on-column inlets are especially useful for trace analysis of thermally labile analytes, especially where an inert inlet is required. The greatest strength of an on-column inlet, that the entire sample that exits the syringe enters the column, is also its greatest weakness. If the entire sample enters the column, so do any nonvolatile matrix components that can easily foul the column after only a few injections.
Besides the possibility of column fouling, the other major drawback of on-column inlets is the syringe. Traditional syringes may be used with 530-µm i.d. megabore columns; however, columns with smaller inside diameters require special syringes with tapered needles or fused-silica needle extensions that can be very fragile and difficult to handle.
Figure 4 illustrates the components of a typical on-column inlet. Similar to other inlets, during an injection the syringe passes through a septum. A needle guide ensures that the syringe needle is properly lined up with the column so that the needle can pass into the column without catching or bending. In a manner similar to a packed column inlet, carrier gas passes around the outside of the column, then through the needle guide and into the column. A septum purge is used to keep the septum clean, which is especially important because the inlet is cooled most of the time. A liquid sample leaving the syringe is depicted on the right side of Figure 4. As the liquid exits the syringe, it will coat the first several centimetres of the inside of the column. As the column temperature is raised, the liquid will be removed in a manner similar to the solvent effects in a splitless injection.
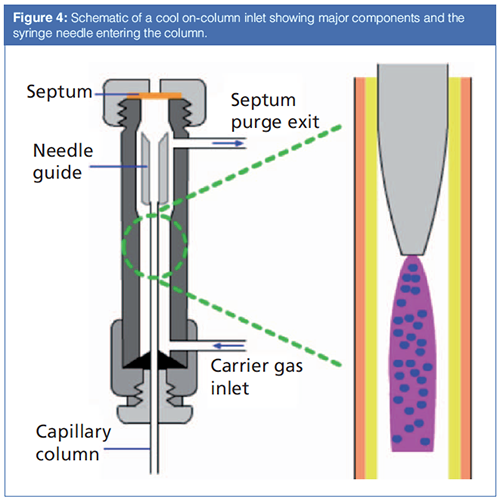
Addition of a precolumn followed by a tee connector with outlets to the analytical column and an external vapour exit adds solvent vapour exit capability that can allow an on-column inlet to be used with large-volume injections up to several hundred microlitres, with the accompanying improvements in detection limits (10).
The advantages of a cool on-column inlet are clear, because the sample is injected without heating. This approach allows samples to exit the syringe without undergoing losses resulting from needle heating, and it deposits the entire injected sample into the column with no losses caused by the heated inlet and glass sleeve. Simplicity and low carrier-gas usage are additional advantages. An on-column inlet is the inlet of choice if inertness and trace analysis are analytical requirements. The major disadvantage is that the column is directly exposed to the entire sample, so “dirty” samples may quickly foul the column, resulting in much greater need to trim or replace columns.
In 1979, Vogt and colleagues modified a split–splitless inlet, similar to the one shown in Figure 1, to allow for rapid heating and cooling of the glass sleeve (11,12). In short, they removed the high-thermal-mass block containing the glass sleeve and replaced it with a low-thermal-mass tube wrapped in heating tape. This modification allows the inlet to be operated in four modes: traditional hot split and splitless plus cold split and splitless. In the two cold modes, injection is followed by rapid heating of the inlet following injection, which transfers the sample to the column under much more controlled conditions than in traditional hot split and splitless modes. This inlet and process, PTV, is highly versatile but requires additional training and method development to use effectively. The PTV inlet became widely available in the 1990s, when it generated considerable attention in the literature. It is still available today, but it has not yet become popular, perhaps because of its additional cost and complexity.
Schematically, a PTV inlet is similar to the split–splitless inlet shown in Figure 1. The low thermal mass surrounding the glass sleeve allows rapid heating and cooling, and allows multiple modes of operation:
- Hot split is the same as traditional split.
- Hot splitless is the same as traditional splitless.
- Cold split and splitless involve injecting the sample under split or splitless conditions into a cooled inlet, typically into a packed or baffled glass sleeve. This technique allows the sample to eject from the syringe and land on the surfaces inside the inlet while cool, avoiding the evaporative effects similar to those shown in Figure 3. The inlet is then rapidly heated to drive the sample into the column. These techniques can be especially useful for thermally labile or sensitive analytes because the sample heating rate can be controlled. Cold split and splitless also reduce or eliminate sample losses resulting from vapour overload and syringe needle discrimination.
- Cold splitless solvent vent or largeâvolume injection is a variant of the cold splitless technique that allows injection of much larger sample volumes. A volume of up to hundreds of microlitres is injected into a cool packed glass sleeve. The packing serves to hold the liquid in place while the solvent is evaporated by carrier gas flow to a vent. There is no flow from the inlet to the column during this evaporation process. After about 95% of the solvent has evaporated, the remaining sample, including the now-concentrated analytes, is transferred to the column under splitless conditions.
On most gas chromatographs, a PTV inlet is not standard equipment. It offers the advantages of versatility, reduction or elimination of the contamination and discrimination problems that challenge split and splitless, and large volume injection capability. The main disadvantage is that it is not as simple to operate. Method development for large-volume injections involves several steps. There are several introductory guides and application examples freely available online (13).
Classical heated split and splitless inlets and injection techniques have served gas chromatographers well for nearly 50 years. However, these inlets are heated continuously and strongly, and their effectiveness for sample introduction into capillary columns is limited. Heating of the inlet throughout the injection process causes several problems, including unnecessary sample losses, band broadening, and possible contamination. In hot split and splitless injections, the amount of sample actually reaching the column can only be estimated. These challenges make a case for gas chromatographers to explore cool on-column and PTV injections. Cool on-column and PTV have been available for almost as long as split and splitless, but have not been nearly as widely used. The several possibilities for cool on-column and PTV described here are only beginnings.
References
- http://www.chromacademy.com/gc-sample-introduction-best-practice.html (accessed May 2018).
- “The Essentials,” LCGC Europe31(5), 298 (2018).
- K. Grob, Split and Splitless Injection for Quantitative Gas Chromatography: Concepts, Processes, Practical Guidelines, Sources of Error (John Wiley & Sons, New York, New York, USA, 2007).
- L. Bai, J. Smuts, P. Walsh, C. Qiu, H.M. McNair, and K.A. Schug, Analytica Chemica Acta953, 10–22 (2017).
- https://www.agilent.com/en/support/gas-chromatography/gccalculators (accessed May 2018).
- http://www.restek.com/images/calcs/calc_backflash.htm (accessed May 2018).
- N.H. Snow and P. Okeyo, J. High Res. Chromatogr.20, 77–81 (1997).
- P. Okeyo, “Solid Phase Microextraction: Optimization and Interface with GC/MS for Trace Analysis of Steroids in Biological Fluids,” PhD Dissertation, Seton Hall University, USA, 1997, pp. 63–83.
- N. Snow, “What Really Happens in the Glass Liner,” chromedia.org; http://chromedia.org/chromedia?waxtrapp= wlqdcDsHonOvmOlIEcCvBC& subNav=bcqpckDsHon OvmOlIEcCvBCuF (accessed May 2018).
- J. Teske and W. Engewald, TrAC, Trends Anal. Chem. 21, 584–593 (2002).
- W. Vogt, K. Jacob, and H.W. Obwexer, J. Chromatogr. A174, 437–439 (1979).
- W, Vogt, K. Jacob, A-B. Ohnesorge, and H.W. Obwexer, J. Chromatogr. A186, 197–205 (1979).
- https://www.glsciences.eu/html/ptv-injection.html (accessed May 2018).
Nicholas H. Snow is Founding Endowed Professor in the Department of Chemistry and Biochemistry at Seton Hall University, USA. He is also the university’s Director of Research and Director of the Center for Academic Industry Partnership. During his 30 years as a chromatographer, he has published over 60 refereed articles and book chapters and has given over 200 presentations and short courses. He is interested in the fundamentals and applications of separation science, especially GC, sampling, and sample preparation for chemical analysis. His research group is very active, with ongoing projects using GC, GC–MS, two-dimensional GC, and extraction methods including headspace, liquid–liquid extraction, and SPME.
“GC Connections” editor John V. Hinshaw is a Senior Scientist at Serveron Corporation in Beaverton, Oregon, USA, and a member of LCGC Europe’s editorial advisory board. Direct correspondence about this column to the author via e-mail: LCGCedit@ubm.com

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.











