Split Injection GC: The Benefits of “Shoot-and-Dilute” GC
Jack Cochran’s new column “Practical GC” aims to provide readers with practical advice and new experimental evidence for how to get the best results from their gas chromatography (GC) systems. The first article in a series on split injection GC focuses on the advantages of using “shoot-and-dilute” GC.
Jack Cochran’s new column “Practical GC” aims to provide readers with practical advice and new experimental evidence for how to get the best results from their gas chromatography (GC) systems. The first article in a series on split injection GC focuses on the advantages of using “shoot-and-dilute” GC.
When I first got into gas chromatography (GC) while working at the US EPA back in the early 1980s, almost all my work as an environmental chemist involved splitless injection, since we were mainly focused on trace-level analyses of pollutants. I learned early on that good splitless injection was not trivial, especially when analyzing dirty samples, and that frequent GC inlet and column maintenance was very important to achieving high data quality. Although I still do trace-level GC analyses for food safety and environmental samples, some of that work now involves split injection, something I like to call “shoot-and-dilute” GC, a play on words that pays respect to the principle of “dilute-and-shoot” liquid chromatography coupled to tandem mass spectrometry (LC–MS–MS). With LC–MS–MS the major problem in producing accurate, quantitative data typically occurs post-column in the ionization stage. Dilute-and-shoot LC–MS–MS is a way to avoid target-compound ion suppression or enhancement associated with complex matrices by substantially diluting the sample prior to injecting it for analysis. Since in GC the main difficulties start at the inlet, “dilution” of the sample by split injection not only puts less sample stress on the GC column, but also shortens the time that sensitive compounds have to interact with the GC inlet liner surfaces and seals. Below is the first part of my series on using split injection to improve GC analyses. Split injection with capillary gas chromatography (GC) has typically been used when analyzing neat or concentrated samples. Examples include petroleum mixtures, essential oils, flavours, and fragrances. Split injection involves introducing only a small portion of the sample to the GC column, mainly to avoid overloaded peaks that corrupt the separation efficiency of the column. Other benefits of split injection, which in some applications are at least as important as not exceeding the sample loadability of the GC column, are: 1. Introduction of a very narrow band of sample to maintain GC column separation efficiency, especially for more volatile peaks. 2. Encouragement of highly efficient transfer of lower volatility compounds to the GC column. 3. Avoidance of sensitive compound degradation through increased inlet liner flow, or maybe better put, a decrease in residence time of compound in an inlet liner that contains glass wool or other potentially reactive surfaces such as liner wall and/or inlet seal. 4. Reduction of adsorption of active components (for example, some pesticides, phenols, or amines) to glass wool or liner wall. 5. Mitigation of late-eluting compound loss (for example, some polycyclic aromatic compounds) to liner wool during analysis of samples containing non-volatile “dirt”. 6. Introduction of less non-volatile “dirt” to the GC column to avoid column performance issues, including compound degradation and peak tailing. 7. Higher GC oven start temperatures to decrease oven cool-down times and increase throughput. 8. Circumvention of sample solvent and GC stationary phase mismatches that lead to bad peak shapes, especially with polar solvents. Split injection points 3–5 above –
less compound degradation, less adsorption of active compounds, and less loss of late-eluting, involatile compounds of interest
– can be contrasted against splitless injection where the flow through the GC inlet liner is much slower, typically in the 1–2 mL/min range. Slow flow through the GC inlet and the requisite longer sampling time for splitless injection offers more opportunity for compound degradation and adsorption, which means poor transfer from the inlet liner to the GC column leading to inaccurate and non-repeatable results. To avoid these problems associated with splitless injection, especially when analyzing dirty samples, more frequent maintenance of the GC inlet, including liner and seal changes, is needed. In addition, removing a few cm of the inlet side of the GC column may be necessary since non-volatile material can collect there and cause peak tailing and reduced response. Both lead to fewer samples analyzed for each monetary unit through GC downtime and consumable costs.
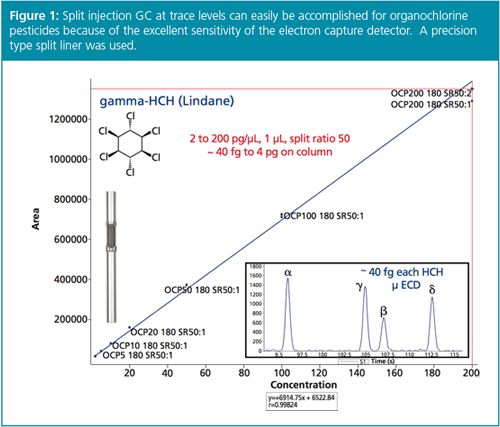
In the past, split injection has presupposed concentrated or neat samples so that compounds of interest could be detected and quantified as repeatable, significant GC peaks. Trace analyses for environmental and food residue work, for example, would fall to splitless injection. However, another way to obtain the benefits of split injection listed above while still accomplishing low-level compound analysis is to use an ultra-sensitive detector. The electron capture detector (ECD) is an excellent case in point, given that it can detect compounds like organochlorine pesticides down to the low femtogram level (Figure 1). Often, extracts of water, waste, soil, or sediment can be easily analyzed using split injection GC–ECD while meeting the desired limits of detection (LODs) and limits of quantification (LOQs). Some organochlorine pesticides, for example, endrin and DDT, are subject to degradation during hot splitless injection, especially when analyzing dirty samples (Figure 2): split injection through high inlet flow and short residence/reaction time can keep pesticide degradation to a minimum (Figure 3), thus increasing the overall GC system uptime.
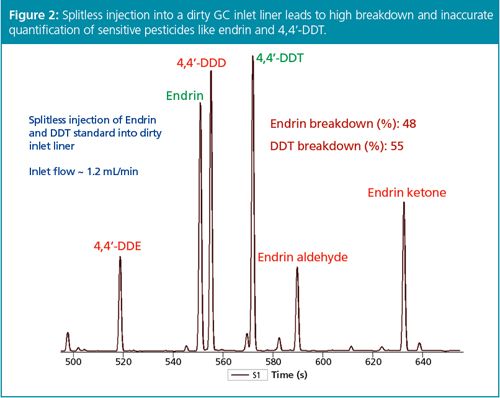
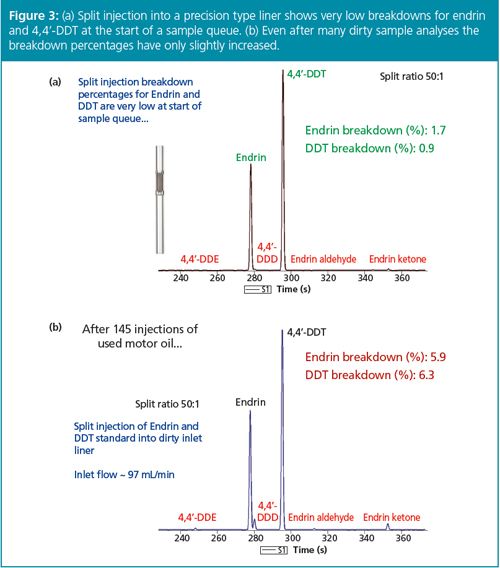
While the ECD is a legitimate example of the end of the GC column enhancing the front of the GC column, through both sensitivity and split injection, many environmental and food residue chemists are using GC coupled to mass spectrometry (MS) and GC–MS–MS because of its sensitivity
and
selectivity. Great strides in sensitivity in GC–MS–MS have been made, allowing users to consider split injection to achieve the earlier stated benefits.
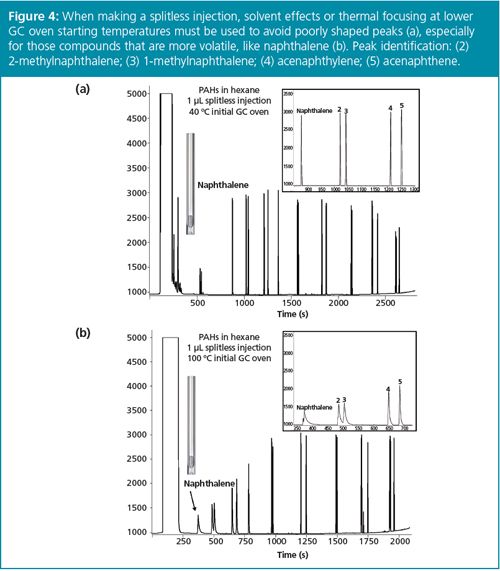
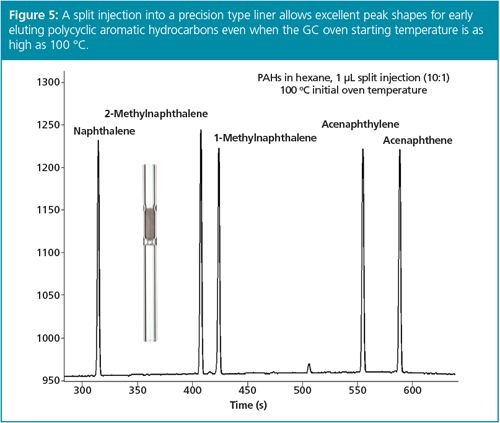
If we can assume that enhanced sensitivity is available for our application, let’s look at a few of the split injection benefits in a bit more detail. Splitless injection requires either solvent effects or thermal focusing (sometimes referred to as stationary phase focusing or cold trapping) to focus analyte peaks during injection to allow efficient GC. Lower GC oven starting temperatures are required, especially when more volatile compounds are analyzed, otherwise peaks are broad and tailing (Figure 4). On the other hand, split injection, even with a split ratio as low as 10:1, allows much higher GC oven start temperatures with good peak focusing, including for volatile compounds (Figure 5). Column efficiency is maintained and sample throughput is increased by not having to cool the GC oven close to ambient temperature.
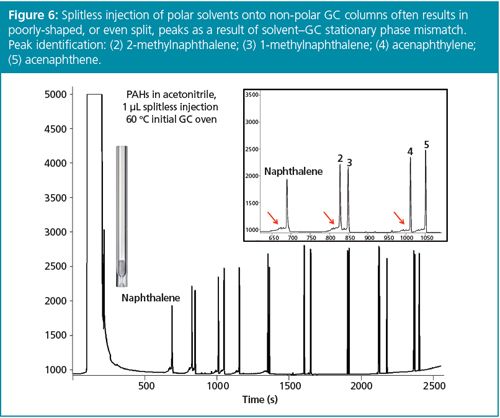
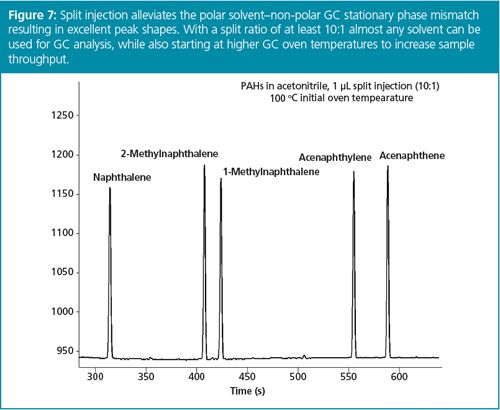
Another major benefit of split injection is that it allows almost any sample extraction or dilution solvent to be used for GC without tedious solvent exchange procedures that might lead to loss of analytes. For example, most GC column stationary phases in use for semivolatile compound analysis are non-polar. Splitless injection of polar solvents onto these phases leads to peak splitting, loss of GC column efficiency, difficulty in peak integration, and lower detectability. As seen in Figure 6, splitless injection of the QuEChERS sample preparation method solvent acetonitrile onto a non-polar GC column causes peak splitting, especially for early eluting analytes. Split injection, however, mitigates this sample solvent–GC stationary phase mismatch to produce excellent peak shapes (Figure 7).
Hopefully this article has encouraged you to try shoot-and-dilute (split injection) for your GC work by presenting some of the practical benefits from its use. To encourage you further, I’d like to end with some tips for easy split injection. • Use a precision type liner packed with wool that wipes the needle during injection. Wool improves homogenization to encourage accurate and repeatable sample transfer. • Start with a split ratio of 10:1 and adjust higher if you can still meet your LODs and LOQs. Having a split vent flow of less than 10 mL/min though can lead to poor repeatability for split injection. • Use the fastest autosampler injection speed to reduce syringe needle discrimination. A slow injection speed allows solvent to evaporate from the needle, which favours transfer of volatile components. • Moderate GC inlet temperatures of 250–300 °C are suggested. Higher flow during split injection helps with quantitative transfer of even relatively non-volatile compounds. • If higher sample throughput is desired, start at the highest GC oven temperature possible that does not increase peak widths for the most volatile target compounds or decrease chromatographic separations for critical compounds.

Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.
Quantifying Microplastics in Meconium Samples Using Pyrolysis–GC-MS
March 26th 2025Using pyrolysis-gas chromatography and mass spectrometry, scientists from Fudan University and the Putuo District Center for Disease Control and Prevention detected and quantified microplastics in newborn stool samples.












