The Smell of Success: Using GC–MS–MS for Low Level Allergenic Fragrance Detection
The regulatory and practical issues that surround allergenic fragrance use within cosmetics and cleaning products are explored in this article.
The regulatory and practical issues that surround allergenic fragrance use within cosmetics and cleaning products are explored in this article. Experimental data is presented to illustrate how recent advances in gas chromatography coupled to tandem mass spectrometry (GC–MS–MS) delivers robust trace analyte quantitation while simplifying method development and reducing the analytical cycle.
Many of the fragrant compounds commonly added to improve the desirability of cleaning agents and personal care products (PCPs) are classified as allergenic. Quantifying the risk of these components to personal safety is complicated because even sub-ppb levels may invoke a serious immunogenic response in hypersensitive individuals. For this reason EU Directive No. 76/768/EC stipulates that a number of allergens should be labelled if they exceed 0.001% (w/w) for rinse-off products and 0.01% (w/w) for stay-on products.1 However, detecting residues at these ultra-low levels presents a number of analytical challenges. The analytical technique must be capable of delivering high performance with excellent sensitivity for trace-level quantitation. Furthermore, developing robust, accurate, and fit-for-purpose methods is an expert task, as is running the screening procedure itself. This makes quality control (QC) and labelling a resource-intensive process. Cosmetics developers therefore require analytical techniques that combine accurate and reliable quantitation and detection with simplified method development. In this article the regulatory and practical issues that surround allergenic fragrance use within cosmetics and cleaning products are explored.

Photo Credit: Nicholas Eveleigh/Getty Images
It is estimated that between 1–3% of the population in Europe have a skin allergy to fragrances.2 Symptoms of allergenic response range from dermatological irritation, swelling, and rashes through to more chronic conditions in hypersensitive individuals, such as eczema. The nature of the reaction to a substance is influenced by a number of personal factors, such as genetic predisposition or age. The level of exposure also impacts the severity of response, although this too may vary between individuals. As a result of these inconsistencies in sensitization, intolerance and (pseudo)allergenic response between hypersensitive consumers regulating the use of allergenic components within cosmetic or personal care products (PCPs) is a complicated issue.
Where public health is concerned most regulatory agencies understandably err on the side of caution. EU Directive No. 76/768/EEC Annex II1 includes a list of substances that are completely prohibited within cosmetics. Where the regulation is less clear cut, however, is in the use of components that are prohibited above certain concentrations. Annex III to the EC directive lists over 250 components subject to such restrictions. Among these are a number of fragrances ranging from relatively obscure compounds through to routinely used fragrances, such as d-limonene, the aromatic responsible for citrus aromas.3
Where compounds listed in Annex III are deployed, the burden of responsibility is on the manufacturer or importer to ensure that these concentration limits are met and that the presence and quantity of these components are clearly labelled. Here, precise quantification at ultra-low concentration is essential.
A New Approach to Analysis
Gas chromatography (GC) coupled with single quadrupole mass spectrometry (SQ–MS) using selective ion monitoring (SIM) is commonly used for residue detection. However, this aproach can produce low signal-to-noise ratios (S/N) and a drop off in sensitivity at low detection levels, particularly in matrix samples.4 GC coupled with tandem mass spectrometry (GC–MS–MS) in multiple reaction monitoring (MRM) mode can provide improved specificity and selectivity down to sub-ppb levels.4
MRM methods of mass spectrometry use a mass filtering function to enhance the sensitivity and selectivity of analyte detection. Ions of interest are first pre-selected as precursor ions based on their molecular weight before undergoing fragmentation within a collision cell. By filtering only the compound-specific fragment ions, MRM MS–MS allows the high selectivity for trace analyte detection and quantitation (Figure 1). This further fragmentation and targeting of multiple MRMs also delivers greater specificity and reduces the likelihood of isobaric interference.
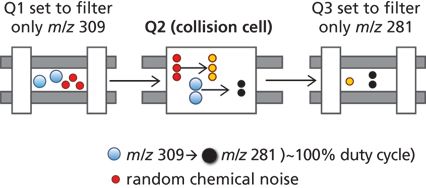
Figure 1: MS–MS MRM monitoring allows complete analyte selectivity.
Developments in instrument hardware continue to improve the sensitivity of MS–MS technology. Achieving high performance throughput while also maintaining robustness typically requires expert operation and lengthy method development, for any complex samples such as food.
To this end, some of the most significant advances in MS–MS technology over the last few years have arisen from the need to deliver simplified method development and faster analysis times.
Case Study - Detecting Allergenic Fragrances in Commercial Products
A typical chromatographic run is divided into a number of segments, and only the group of analytes eluted in each segment are monitored at one time. However, analytes may elute in the junction between two segments and avoid detection. To ensure against this, MRMs need to be carefully set up to catch these species and multiple MRM traces are required to confirm each residue. This will provide confidence in the reliability of the data required for QC and product labelling, but it also makes developing a method time consuming. Poor method development at this stage of the MRM screening process can lead to poor sensitivity and precision.
Recent developments in MS–MS software provides a different approach to MRM screening. Rather than targeting each individual MRM, advanced screening software can focus on the compounds themselves.
Method: 25 cosmetics from local and foreign retail markets were mixed with 27 potentially allergenic compounds. The detergents and cleaning agents within the samples were extracted in a water bath at 55 °C in the presence of an internal standard. Samples were spiked with an internal standard and diluted 1:1000. Solid-phase extraction (SPE) was performed on a octadecyl carbon chain (C18) column packing material (Restek). Samples underwent analysis with a a GC system connected to tandem MS system. Experimental details were as follows:
GC–MS–MS: Bruker GC 456 Scion TQ; S/Sl-injector temperature: 250 °C; split: 1:20; column: BR-5ms, 30 m × 0.25 mm, 0.25-μm; oven programme: 50 °C (1 min), 250 °C (12 °C/min); 3.33 min; injection volume: 1 μL; MRM measurements with optimized transitions for all compounds (at least two transitions for each compound); data system: MSWS 8.0 SP2 (Bruker).
The software used contained a spectral library of over 2500 MRM transitions, including the majority of fragrances listed within the EC legislation (Compound Based Scanning [CBS] software [Bruker]). Each compound in this library is linked to a number of characteristics that define its performance during analysis, including retention time, primary and secondary MRM transitions, and collision energy. By using this data the system automatically builds a screening method for the target analytes and optimizes the dwell time.
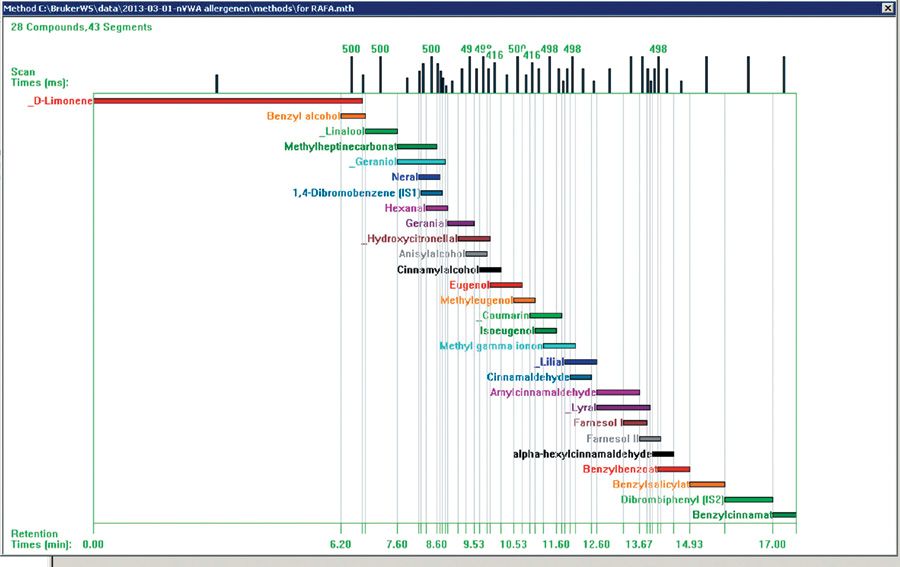
Figure 2: Reference spectra calculates optimal scan times for method development.
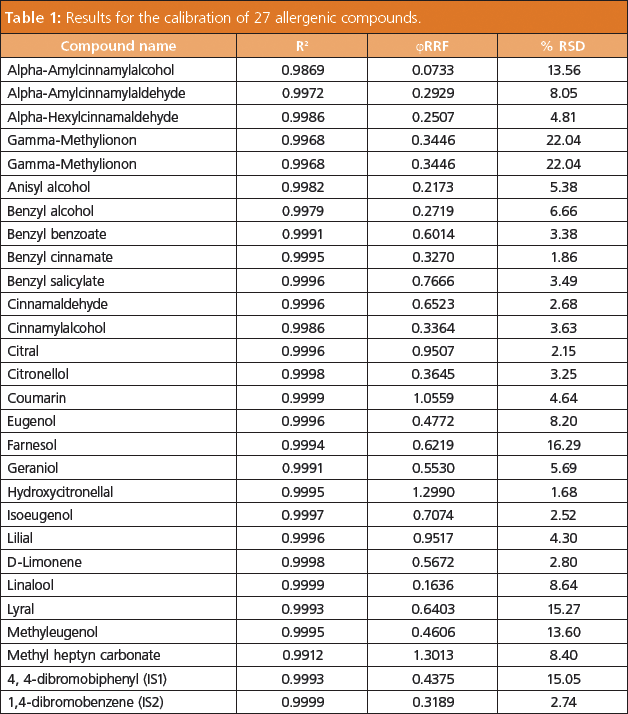
Results: After a small number of initial runs to locate the retention time window for each compound, the software selected optimal scan times for the 27 allergen compounds (Figure 2). The MS workstation automatically calculated and assigned the scan time for each MRM. Following sample analysis, a data table was automatically populated with MRM information obtained from the acquisition method. The acquired data was then reviewed and retention times or ion ratios from the experiment adopted into the acquisition method for subsequent samples. For the compounds listed in Table 1, calibration curves from 5 ppt to 250 ppb could be measured, satisfying the detection limits required for cosmetic labelling. Good signal-to-noise ratio was achieved using GC–MS–MS. Figure 3 shows an example of a full scan and MRM-trace for a commercially available perfume sample from a retail market. The MRM trace clearly identifies the presence of 10 allergenic compounds.
The enhanced selectivity and specificity of MS–MS is illustrated in Figure 4. Here the ion trace for the analysis of allergenic fragrance anisylalcohol within a sample of vanishing cream is performed with (a) MS–MS and (b) conventional single quadrupole SIM. Vanishing cream is a heavy matrix sample and, as such, SIM techniques struggle to provide clear resolution during trace level analysis. MS–MS on the other hand produces a clearly defined peak with a retention time of around 9.6 min indicating excellent selectivity for the target analyte.
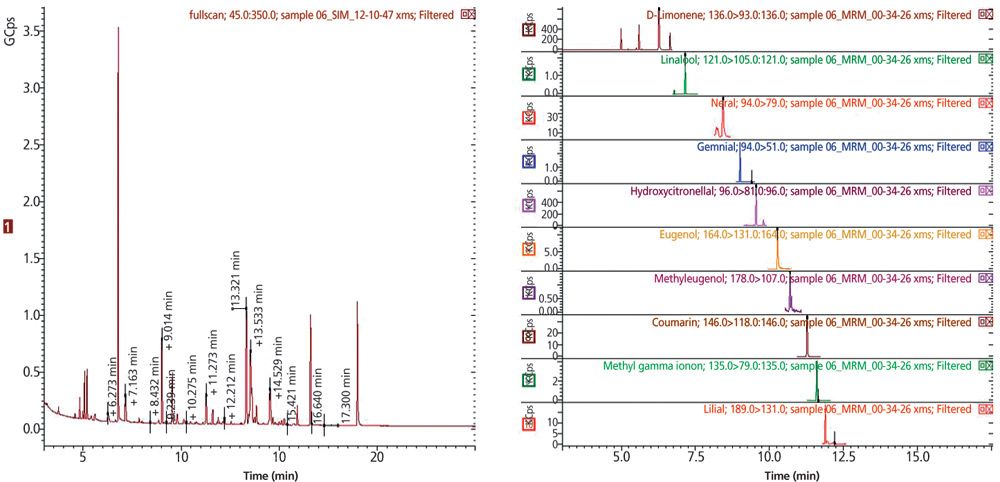
Figure 3: Full scan and MRM trace for a perfume sample measured using GC–MS–MS in MRM mode revealing the presence of 10 allergenic fragrances.
Overall excellent sensitivity, selectivity, and linearity were achieved for trace level fragrance analysis within PCPs and cleaning products using MS–MS analysis in MRM mode.
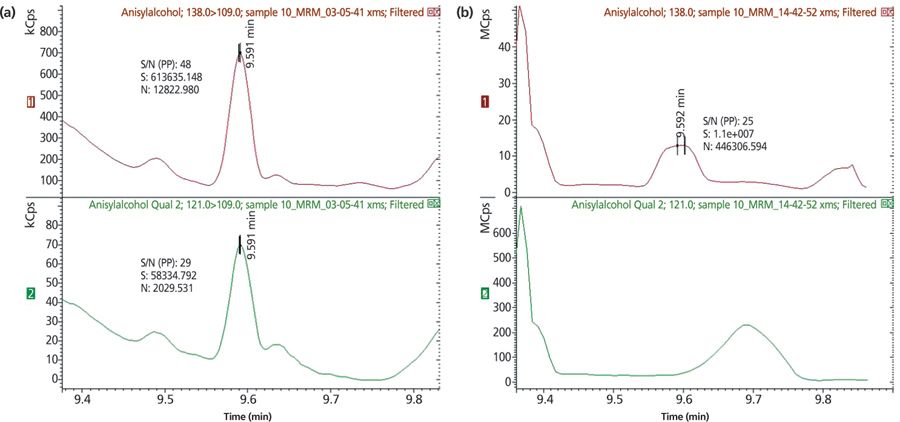
Figure 4: Comparison of exemplary ion trace for target allergen anisylalcohol in vanishing cream with (a) MS–MS and (b) single quadrupole MS.
Summary
Ensuring the safety of the consumer and meeting industrial regulations is a primary focus of cosmetics and personal care product formulation and quality control. The variability in severity of response to allergenic compounds means that accurate labelling of consumer products is vital to safeguard the user. GC combined with tandem mass spectrometry is one of the few analytical techniques with the sensitivity and specificity for accurate trace residue screening in matrix samples. Combined with advances in software, which greatly simplify method development and shorten the analytical cycle, GC with tandem mass spectrometry delivers this high performance along with increased productivity and ease of use.
References
1. Hazardous Substances and New Organisms Act 1996, Cosmetic Products Group Standard 2006, Environmental Risk Management Authority. http://www.epa.govt.nz/Publications/gs-cosmetic.pdf
2. European Commission, Consumer affairs current consultations. http://europa.eu/rapid/press-release_MEMO-14-108_en.htm
3. Table 3. Changes to Fragrance Allergens in Annex III. http://www.cosmeticsandtoiletries.com/articleaddons/46487402.html
4. Jon W. Wong et al., J. Agric. Food Chem.58(10), 5868–5883 (2010).
Joe Anacleto received his Ph.D. in 1993 on the use of various chromatographic mass spectrometric techniques for the analysis of food and environmental contaminants. He has been an active member of the analytical instruments community overseeing the development and marketing of mass spectrometry-based solutions to new and emerging markets. In his current role, Joe is responsible for the continued expansion of Bruker's portfolio of analytical solutions for food, environmental, and forensic testing.
Gordon van 't Slot holds a PhD in food chemistry from the "Westfälische Wilhelms-Universität", Münster, Germany, on the study of the metabolic and microbial fate of xenobiotics. He works as a senior application chemist at "Bruker Daltonik GmbH" for triple quad mass spectrometry. Dr. Gordon van 't Slot has also worked as manager of a QS-accredited pesticide residue analysis laboratory, and has been a lecturer in dietary biochemistry. His interests lie in consumer protection and environmental health.
E-mail: joe.anacleto@bruker.com
Website: www.bruker.com
This article is from The Column. The full issue can be found here>>

Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.











