Six Key Differentiators Between Liquid Chromatography and High-Resolution Ion Mobility
Ion mobility separations are based on fundamental principles that differ from liquid chromatography (LC) in that several parameters of ionized molecules—size, charge, shape, and structure—come into play simultaneously. As a result, analytes that have the same molecular mass can be separated by their shape, charge and collision cross section (CCS). A new development in ion mobility separation, high-resolution ion mobility (HRIM), overcomes the problem of ion loss in other ion mobility separation techniques. HRIM also provides excellent structural resolution and high reproducibility. The technique is particularly well suited to challenging applications in the pharmaceutical and clinical fields, such as glycosylation monitoring of biological drugs and vitamin D analysis.
Truly disruptive advances catapult onto the scientific stage when a convergence between an emerging need and innovation occurs, thus thrusting a new technology into the limelight. High-resolution ion mobility (HRIM) based on structures for lossless ion manipulation (SLIM), originally invented in the laboratory of Dr. Richard D. Smith at Pacific Northwest National Laboratory (PNNL), provides the unprecedented capability to separate and identify molecular structures that are practically indistinguishable using traditional methods such as liquid chromatography (LC). Some of these inherently difficult to detect analyte classes include peptides, proteins, lipids and glycans. In fact, HRIM’s separation capability is powerful enough to separate and detect isobaric molecules; one particularly relevant example of this is glycan analysis of the novel coronavirus spike protein (1).
Classical analytical separation techniques, like LC, are often too slow, too experimentally challenging, or not powerful enough to resolve and structurally characterize molecules to meet the current demands for fast or high throughput commercial applications. HRIM offers an alternative that should be evaluated where appropriate to help meet the demand for fast, efficient analyses, including biologic therapeutic development and biomarker analysis.
What is Ion Mobility?
Ion mobility separations are based on fundamental principles that differ from liquid chromatography, in that several parameters of ionized molecules—size, charge, shape and structure—come into play simultaneously. As a result, analytes that have the same molecular mass can be separated by their shape, charge and collision cross section (CCS).
Ion mobility separation has been available for decades, but there remains concern about ion loss leading to a reduction in sensitivity, the mass range per analysis is limited, and the length of the separation path is not sufficient enough to achieve high-resolution separation. The distinguishing feature of HRIM compared with other ion mobility separation techniques is that separations are achieved essentially losslessly on very long pathlengths implemented with serpentine electrode patterns on conventional printed circuit board (PCB) technology. Digitizing separations, so to speak, on PCBs with nearly limitless pathlengths addresses the ion loss and resolution limitations of incumbent technology. With HRIM technology, ion manipulation and separation are achieved by applying electric fields to electrodes on two parallel PCBs to create an ion conduct through which ions travel without striking physical surfaces and thereby avoiding neutralization and loss that are typical to other ion mobility technologies.
The serpentine separation path design packs a single pass 40-foot ion path (in the first commercial product) into a device about the size of a laptop, addressing the resolution limitations inherent to shorter pathlength ion mobility instruments, whether multi-pass cyclic or linear, and ultimately achieves performance that enables characterizations that were previously impossible. The long pathlengths achieved with HRIM, and the foundational separation mechanism of ion mobility technology, provide additional structural information not otherwise gleaned from other separation techniques.
The Differentiators Between High-Resolution Ion Mobility and Liquid Chromatography Mechanism of Separation
HRIM separates ions based on the difference in collision cross-section, size, charge density and overall shape. The sample of interest is dispersed in the gas phase using conventional electrospray ionization. As the ions enter the separation chamber, voltages applied to electrodes on the PCBs provide the lossless separation conduit through which the ions traverse along the separation pathlength. Separation is achieved because smaller CCS molecules travel faster than the larger CCS molecules and hit the detector first. Because CCS is an inherent physical property, highly reproducible results are achieved as long as pressure and temperature are controlled within the system. With separation occurring in the gas phase compared to the liquid phase with LC, separations and data collection are achieved in milliseconds not minutes or hours. Thus, dozens of samples could be processed in the time required for a single LC run.
Analyte Agnostic: No Column Changes for Greater Instrument Uptimes
“Those LC guys need to understand a bit of magic,” is the way this author has heard it phrased. LC often necessitates matching column size, length, and packing material to specific separations and their intended purposes. In many cases, columns are dedicated to particular types of analytes. For example, one set of protein samples will be run on a different column than a set of small molecule samples. Similarly, an operator might use different column materials (positively or negatively charged, polar or non-polar) to separate different types of molecules. LC very often relies on adjusting the solvent (liquid) system to the task at hand, which may require complicated calculations, trial runs, algorithms for step or gradient changes, and so on. In reality, scientists are running an entirely different set of experiments, each time, for each analyte type, complete with lengthy experimentation to validate the method development.
Scientists who work with LC are often extremely experienced operators, sometimes like a magician in their ability to utilize workarounds and nuances to overcome stumbling blocks and achieve successful results.
In contrast, HRIM allows scientists to resolve multiple classes of analytes without component changes, as there are no hardware changes to make. Less experience is required to run experiments, and often, with less than a week’s worth of training, the experiments can be up and running. In a laboratory setting where different sample types (such as glycans, peptides, proteins, small molecules) are the mainstay, the same HRIM instrument can be used across the board. Running samples of varying analytes back-to-back is practical and simple, significantly increasing laboratory productivity.
Reproducibility
With HRIM, reproducibility and achieving consistent data collection depends less on the experience of the operator. HRIM achieves separation of ionized molecules based on the physical parameters of the analytes themselves.
Since HRIM does not make use of specific columns or solvent systems, and separations are analyte agnostic, there are fewer variables to control, and the process for running HRIM requires much less user input. HRIM technology lends itself to more reproducible results because the separation mechanism is an inherent physical property with fewer variables than LC based methods. Enabling more predictable and more reproducible results across different laboratories, LC’s reproducibility is highly dependent on the preparation of buffers, solutions, and samples, method development, solvent amounts, column, flow rate, and sample size can each affect the reproducibility of the results. In fact, data reproducibility has been the most consistent comment received from HRIM’s beta users.
Higher Peak Capacity and Resolution
To put the comparison simply, the biggest difference between HRIM and LC is the level of resolution that is achievable in a short amount of time. In situations where multiple molecules have similar elution times on the chromatogram, LC separation can take more than an hour to distinguish them effectively, whereas HRIM routinely achieves the most challenging separations in two to five minutes. HRIM eliminates the common tradeoff between speed for resolution or vice versa, resolution for speed. With HRIM you can have both speed and resolution without sacrifice.
HRIM outperforms in areas that have been notoriously challenging: specifically, lipid and glycan analysis. In fact, study of these biomolecular classes has suffered from the difficulty to closely follow their biosynthesis, structural distribution, and metabolism, largely due to isomeric natures and vast structural diversity (2–4).
HRIM has come to the forefront to meet these needs. Biosimilar development provides one example of the importance of glycan analysis, where in order to achieve regulatory approval, the biosimilar glycan profile must match the originator (5). The more detailed the glycan profiles for the purpose of comparison, the higher likelihood of approval.
Collectively, scientists using HRIM can achieve unprecedented glycan separation, including distinct elucidation of isomers and other molecules with highly similar composition. Advances in glycan characterization are more important now, more than ever, given the need to understand glycosylation of human and animal viruses such as influenza, HIV, and coronaviruses. HRIM is currently being used to detail the glycosylation microheterogeneity in the spike glycoprotein that decorates the surface of the SARS-CoV-2 (Covid- 19) viral capsid. It is expected that this information will aid understanding of the heterogeneity in glycosylation on this surface protein, help explain how the virus binds to its target, and aid the development of an effective treatment. This particular application of HRIM makes clear its enormous potential, especially in conditions where full resolution and fast analysis are necessary.
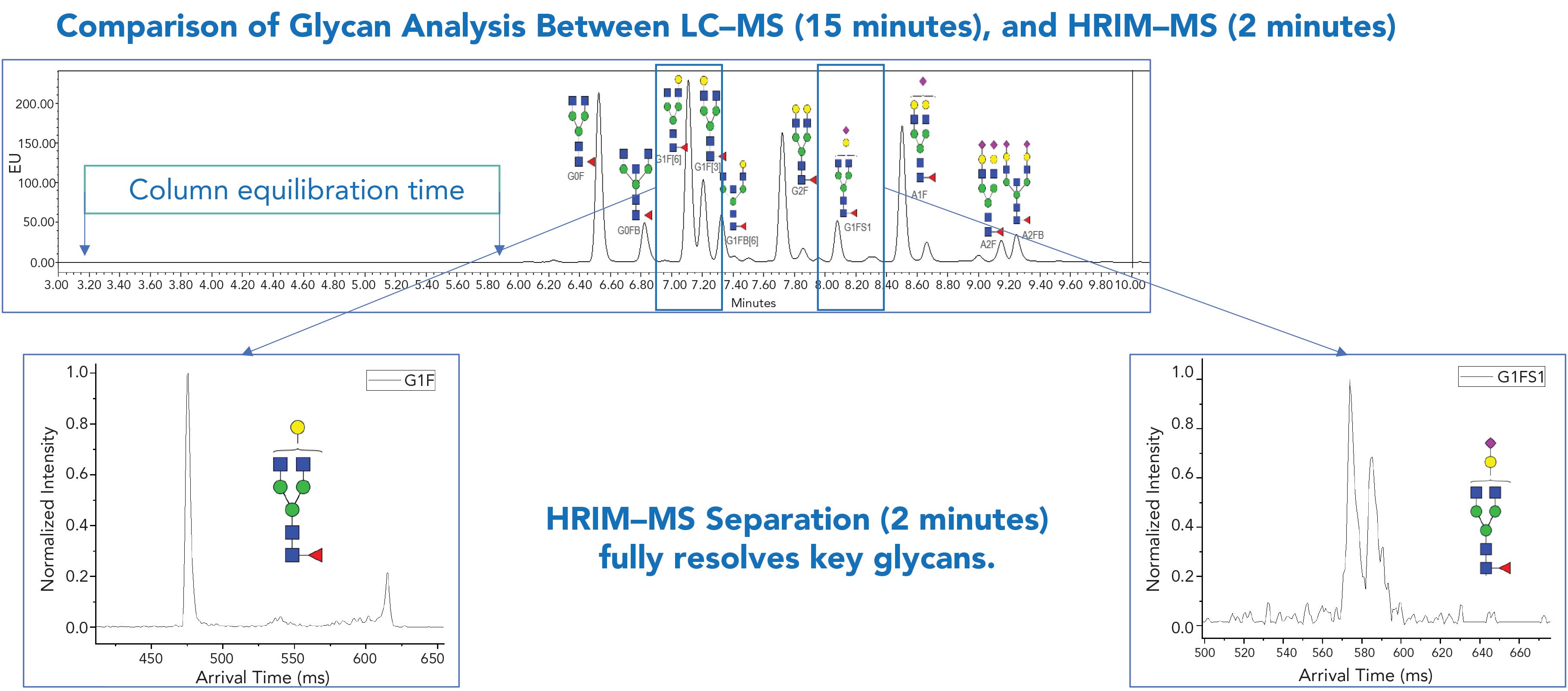
Figure 1: HRIM-MS produces higher quality data more quickly, resulting in savings for development costs and improvement of safety and efficacy for potentially faster regulatory approvals. Shown above, a 15-min LC–MS generated chromatogram of IgG control with N-glycan identifications noted, and the extracted ion mobility drift plots (or mobiligrams) from the 2-min HRIM–MS method demonstrating resolution of glycoforms not identified using the LC–MS method.
Method Development
Method validation is the process of proving that an analytical method is acceptable for use. Verifying that an analysis procedure will accurately and consistently deliver a reliable measurement of an active ingredient in a compounded preparation requires that the method be specific, accurate, and precise over a usable range. Furthermore, it must have an acceptable limit of detection and quantitation, and be robust enough for the demands of the experimental conditions.
Software platforms can assist with method development for LC; however, trial runs and adjustments to a myriad of variables can consume hours, if not days. In the end, the method may not be valid for all sets of conditions, or all the desired analytes.
HRIM method development is driven by software that provides real time visualization of adjustments being made to any preprogrammed workflow. Adjustments are made live, in process, significantly reducing method development time. Method development is not only faster, but methods are more easily and consistently transferred from laboratory to laboratory.
Operator Training Time
While HRIM is based on highly sophisticated chemical and electronic properties, such as collision cross-section and ion polarity, less sophisticated operators can still achieve useful separation results. Generally, scientists are able to run the instrument after only two days of training.
Good sample handling methods, understanding the experimental goals, and overall interpretation of the analytical results are always important, for both LC and HRIM. However, HRIM’s ease of use allows greater focus on interpretation and analytical result evaluation, rather than the grind of running samples.
Conclusions
Liquid chromatography is the laboratory workhorse for straightforward, high throughput applications, and quantitative bioanalysis of analytes of interest.
For some routine separations and analyses, the superior resolving power of HRIM technology may not be required. Still, for other applications, HRIM’s superior resolution, straightforward method transfer, greater instrument uptime, and 5x to 60x faster analysis make it well worth evaluating. Since 2014, more than 35 papers have been published describing the scientific theory behind SLIM, as well as the evidence in support of its adoption as the new gold standard in separations technology.
The most recent publications from 2021 that demonstrate the capabilities of HRIM–MS include work from the McLean group at Vanderbilt University, where near baseline resolution of several biochemical isomers with as little as 0.6% difference in CCS was achieved (6). Another from Dr. Kim Ekroos discusses a 2-minute, LC free method to separate isomeric species from the ganglioside lipidome with superior resolution and reproducibility (7). Finally, a workflow targeting biopharmaceutical characterization that demonstrates a fourfold decrease in chromatographic method times while resolving Post Translational Modifications that were undetected with LC‒MS alone (8). The future of ion mobility has arrived.
Author Note
MOBILion is the exclusive licensee of the SLIM technology for commercialization purposes.
References
(1) Mobilion Systems Inc., “Mobilion Partners with Investigators at the Complex Carbohydrate Research Center at the University of Georgia for COVID-19 Glycan Analysis” (April 2020) https://mobilionsystems.com/ PressReleaseCCRCPartnershipCOVID
(2) J.E. Kyle, X. Zhang, K.K. Weitz, et al., Analyst 141(5), 1649‒1659 (2016). https://doi.org/10.1039/c5an02062j
(3) K.K. Palaniappan and C.R. Bertozzi, Chem. Rev. 116(23), 14277–14306 (2016). https://doi.org/10.1021/acs.chemrev.6b00023
(4) J. Hofmann and K. Pagel, “Glycan Analysis by Ion Mobility–Mass Spectrometry“ (April 24, 2017). https://doi.org/10.1002/anie.201701309
(5) B.L. Duivelshof, W. Jiskoot, A. Beck, J.-L. Veuthey, D. Guillarme, and V. D’Atri, Anal. Chim. Acta 1089, 1‒18, (2019). ISSN 0003-2670, https://doi.org/10.1016/j. aca.2019.08.044
(6) J.C. May, K.L. Leaptrot, B.S. Rose, K.L. Wormwood Moser, L. Deng, L. Maxon, D. DeBord, and J.A. McLean, J. Am. Soc. Mass Spectrom. 32(4), 1126‒1137 (2021). DOI: 10.1021/jasms.1c00056
(7) K.L. Wormwood Moser, G. Van Aken,D. DeBord, N.G. Hatcher, L. Maxon, M. Sherman, L. Yao, and K. Ekroos, Anal. Chim. Acta 1146, 77‒87 (2021), https://doi.org/10.1016/j.aca.2020.12.022
(8) J.R. Arndt, K.L. Wormwood Moser, G. Van Aken, R.M. Doyle, T. Talamantes, D. DeBord, L. Maxon, G. Stafford, J. Fjeldsted, B. Miller, and M Sherman, J. Am. Soc. Mass Spectrom. DOI: 10.1021/jasms.0c00434
Melissa Sherman is CEO of MOBILion Systems, Inc. in Chadds Ford, Pennsylvania. Laura Maxon is the Director of Business Development and Corporate Strategy at MOBILion Systems, Inc. in Chadds Ford, Pennsylvania. Direct correspondence to: info@mobilionsystems.com
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)






