Simplifying Solid-Phase Extraction Method Development: Exploring the Use of Software
Special Issues
SPE is a popular sample preparation technique, valued by analysts working with complex matrices.
Solid-phase extraction (SPE) is often used to clean up complex sample matrices before analysis. Over the last couple of decades, many advances have been made in the development of available phases, the most significant of which are functionalized polymeric SPE sorbents. The combination of traditional silica-based and functionalized polymeric sorbents allows SPE users to take advantage of a number of modes of interaction including reversed phase, normal phase, hydrophilic, and ion exchange. Whether analysts are looking to extract target analytes from a complex matrix, concentrate trace amounts of a specific compound, or perform a solvent switch before injection, the biggest challenge is developing a starting SPE method.

(Photo: Getty Images)
Despite the many benefits SPE has to offer, such as increasing detection sensitivity and protecting the analytical system, the extensive time and resources required to develop a method can be roadblocks to implementing this very effective sample preparation technique. To combat these challenges, the aforementioned SPE method development software takes into account the chemical and physical characteristics of the target analytes and sample matrix information to produce a complete method, including SPE sorbent phase, sorbent mass, load, wash, and elution conditions.
SPE Method Development
Traditional SPE method development starts with a good understanding of the target analyte. Analyte pKa, logP, solubility, and stability are all required for the development of an effective sample pretreatment and a sorbent selection that will allow for the greatest retention of the target analytes under the strongest wash conditions. The optimal sorbent mass is dependent upon the sample matrix and volume. This prevents overloading of the sorbent bed and a loss of analyte. Where the wash and elution volumes are determined by the sorbent bed mass, the solvents and buffers and their respective concentrations are determined for the target compounds. Multiple variables, including retention mechanism, retention strength, compound stability, and compound chemical characteristics, determine the makeup of wash and elution solvents. Collectively, all of these factors form the basis for any reproducible SPE method.
Method troubleshooting and optimization are often the most involved and time-consuming aspects of developing an SPE method. Low-recovery troubleshooting, for example, must be a methodical process starting with the discovery of where the target analytes are being lost. Loss of analyte during SPE is often tied to a wash solvent that is too strong; however, a wash solvent that is too weak will lead to a less effective cleanup of the sample, leaving detection-interfering compounds in the sample. Therefore, identifying balanced and effective wash and elution solvents can be challenging, but both contribute significantly to the success of any SPE method.
Software method development: The software used here (Strata-X SPE Method Development Software, Phenomenex, Torrance, California) uses a two-step process to build an SPE method around target analytes. In the first step, the user enters details about the sample matrix, sample volume, desired elution solvent, and the desired SPE format (96-well plates or tubes). In the second step, the program captures the pH stability range and information about the aromaticity of the main target analyte. The user then can choose three options for entering additional analyte details: define the functional groups present or enter the chemical formula, enter acidic or basic pKa and logP details, and import an MDL (Symyx Technologies, Inc., Sunnyvale, California) file containing structural information for the compound.
With all analyte information entered, the software creates the most ideal SPE method. Although the original method created can yield the desired results, method optimization is often required to achieve the highest possible recoveries. Users are given the option to have the program suggest an alternative method if foreseeable problems are thought to exist upon initial review of the original method suggested, or if users are unsatisfied with results after trying the original method. Regardless of the method that is used, the troubleshooting section pinpoints problems and offers multiple suggestions for improving results, prioritizing the suggestions by what is most likely to resolve the issue.
Method comparison: To test the applicability of this software for use in the development of an actual SPE method, we compared the recommended method developed by the software to an SPE method developed in the laboratory for the extraction of benzalkonium chloride out of plasma. Benzalkonium chloride was chosen primarily because it contains a quaternary amine and because of the unique challenges that functional group presents when performing an extraction based upon an ion-exchange mode of interaction (see Figure 1). Aside from the chemical properties of the compound, benzalkonium chloride has practical applications, including biocidal properties, specifically with the C12–C14 alkyl derivatives.
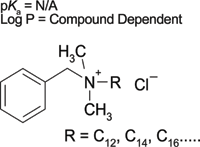
Figure 1: Chemical structure of benzalkonium chloride compounds.
The positive charge held by benzalkonium chloride, due to the quaternary amine functional group, is independent of pH and cannot be neutralized. As a result, a strong cation-exchange SPE sorbent cannot be used because it will lead to the irreversible binding of the compound during extraction. A weak cation-exchange sorbent is a better alternative to exploit the strong retention mechanism that ion-exchange materials have to offer and to elute the positively charged compound by neutralizing the sorbent.
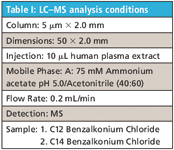
Table I: LCâMS analysis conditions
Laboratory-developed method: The SPE extraction method that was developed manually in the laboratory, without the use of the SPE software program, looked at plasma samples spiked with benzalkonium chloride at a concentration of 100 ng/mL. Strata-X-CW (Phenomenex, Inc.), a weak cation-exchange, functionalized polymeric sorbent, 30 mg/1 mL SPE tube, was used to perform the extraction. The cartridge was conditioned using 1 mL of methanol and equilibrated with 1 mL of water. The sample loaded onto the cartridge was made up of 350 μL of spiked plasma and 700 μL of water acidified with 20 μL of phosphoric acid at a pH around 7. A first wash consisting of 1 mL of 25 mM sodium acetate buffer at pH 4.5 was passed through the cartridge, followed by a second wash consisting of 1 mL methanol. The cartridge was dried for 1 min by vacuum. Elution was performed using 1 mL of 5% formic acid in methanol. The elution fraction collected was evaporated to dryness and reconstituted in the mobile phase used for the subsequent liquid chromatography–mass spectrometry (LC–MS) method.
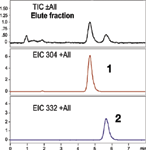
Figure 2: LCâMS chromatograms showing the separation of benzalkonium chloride C12 and C14.
LC–MS analysis was used to analyze the extracted plasma sample. The isocratic LC–MS method, shown in Table I, utilized a Luna CN column (Phenomenex, Inc.). The LC method results in the separation and elution of benzalkonium chloride C12 and benzalkonium chloride C14 in less than 7 min (see Figure 2). A calculated absolute recovery for benzalkonium chloride C12 was 85% and for benzalkonium chloride C14 was 86% (See Table II). Over four extractions, the relative standard deviations (RSD) for both compounds were less than 10%.

Table II: Results (absolute recovery)
Software-Developed Method
Using the SPE method development software, details about the sample and target compounds were entered. The sample details included plasma sample matrix, sample volume of 0.25 to 0.5 mL, and the SPE format used. Compound details that were entered included aromatic compound, pH stability from 1–12, and the presence of a quaternary amine group. Because there is little difference between benzalkonium chloride C12 and C14, the method details were entered for only one analyte. With those details entered, the SPE method created contained the following details:
- SPE product: Strata-X-CW 100 mg/1 mL
- Conditioning: 1 mL methanol, use flow rates up to 3–6 mL/min
- Equilibration: 1 mL buffered solution pH 7, use flow rates up to 3–6 mL/min.Recipe for 50 mM pH 7 buffer: 6 g of NaH2PO4 in 900 mL of water; titrate to pH 7 with KOH/NaOH and HCl as required; make up to 1 L with water
- Sample load: 0.25-0.5 mL of plasma diluted with 2 parts pH 7 buffer, use flow rates up to 1–2 mL/min
- Wash: 1 mL water, use flow rates up to 1–2 mL/min
- Wash: 1 mL methanol, use flow rates up to 1–2 mL/min
- Elute: 1 mL 2% formic acid in 1:1 methanol–acetonitrile, dry cartridge for 1 min under full vacuum before applying elution solvent, allow elution solvent to soak into sorbent for 0.5–1 minute, use flow rate up to 1–2 mL/min
Method Comparison
When the manually developed SPE method is compared with the one developed by the software, few differences are seen. Differences seen in the suggested format, 100 mg/1 mL versus 30 mg/1 mL can be accounted for based upon the fact that the software will err on the side of caution and assume a 0.5-mL sample load, the higher end of the sample volume range entered. Suggested cartridge conditioning, equilibration, and load steps are the same between the two methods. The manually developed method calls for an acidified first wash, however, if yields are affected adversely by a water-only wash, as suggested in the software method, troubleshooting with the software would lead to the suggestion of acidifying the wash step. The final difference between the two methods is in the elution step, where the manually developed method utilizes 5% formic acid in methanol and the software developed method calls for 2% formic acid in 1:1 methanol–acetonitrile. A lower concentration of formic acid, as suggested in the software method, might not be enough to neutralize the sorbent phase and allow for the release of the quaternary amine, but this would be addressed by the troubleshooting feature built into the software program.
Accepting that method optimization is always required, the software is an effective tool in SPE that can save developers a significant amount time compared to a manually developed method.
Jeremy S. Bierman and Michael V. Campognone
Phenomenex, Inc., Torrance, CA
Please direct correspondence to jeremyb@Phenomenex.com

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.













