A Simple and Solvent-free Method for Determining Tetracyclines in Prawns
Reducing or, if possible, eliminating the use of organic solvents is an important goal in terms of environmental conservation, human health and the economy.
Tetracycline antibiotics (TCAs) are used globally in aquaculture to control disease and promote growth. The tetracyclines used in veterinary medicine in Japan account for more than half the total of antibiotics used.1 The overuse of TCAs can result in their presence in seafood for human consumption.

This is a serious problem in Japan where seafood is consumed in copious amounts and prawns are the Japanese consumer's favourite choice. A report that 2.3 ppm of oxytetracycline (OTC) was detected in imported Japanese Tiger Prawn (Marsupenaeus japonicus) magnified these concerns. This level is greater than the minimal risk level (MRL) of 0.1 µg/g in fish — singly or in combination — the EU has set for OTC, chlortetracycline (CTC) and tetracycline (TC).2 Correspondingly, the Codex MRL for OTC is 0.2 µg/g for Black Tiger Prawn (Penaeus monodon),3 which is consistent with the value set for fish by the Japan Ministry of Health, Labor, and Welfare.4
The EU, Japan and the US import more than 70% of the world seafood market5 and it is essential to monitor the safety of seafood for consumers in relation to the presence of tetracyclines.
The ideal method must be simple, quick, cost-effective and cause negligible harm to the environment and the analyst. High performance liquid chromatography (HPLC) is now the preferred method for analysis of many substances, including TCAs of biological interest.6 HPLC interfaced with ultraviolet (UV), fluorescence (F),8,9 photo-diode array (PDA),10–12 and mass spectrometery (MS) detection,7,13 have been reported. MS is very expensive and is not available in a lot of labs for routine analysis, particularly in developing countries.
Environmental Impact
The methods mentioned in references 8–13 share crucial disadvantages. They all consume organic solvents in the HPLC mobile phases and in the solutions used to extract and deproteinize the sample during the sample preparation stage.The disposal of organic solvents is a global environmental problem in terms of risk to humans and environmental impact.4–17
Incineration of waste organic solvents has steadily increased over the past 10 years but is very expensive.18,19 Reducing or, if possible, eliminating the use of organic solvents is, therefore, an important goal in terms of environmental conservation, human health and the economy.
All these techniques also involve at least one extracting/purifying step using large amounts of organic solvent, which consumes time and money if a large number of samples need to be analysed. Additionally, most of these methods did not detect OTC, CTC and TC simultaneously. This article describes an inexpensive technique that does not use organic solvents to determine OTC, CTC, TC in prawns. This involves a fast sample preparation technique followed by RP-HPLC interfaced with a PDA detector using 100% aqueous conditions for the mobile phase and sample preparation stage.
Experimental
Procedure: Black Tiger Prawn tissues were minced fully and used as the blank samples. An accurately weighed 0.1 g sample was taken into a 1.5 mL microcentrifuge tube and homogenized with a hand-held ultrasonic-homogenizer (model HOM-100, 2 mm i.d. probe, Iwaki Glass Co. Ltd, Funabashi, Japan) for 30 s with 0.6 mL of a 20% (w/v) trichloroacetic acid (TCA) solution. After being homogenized, the capped tube was centrifuged at 12000 g for 5 min. The supernatant liquid was filtrated through the 0.45 µm filter unit and the filtrate was then injected into an HPLC system.
HPLC: The HPLC system included a model PU-980 pump and DG-980-50 degasser (Jasco Corp., Tokyo, Japan) equipped with a model CO-8010 column oven (Tosoh Corp., Tokyo, Japan), as well as a model SPD-M10A VP PDA detector (Shimadzu Scientific Instruments, Kyoto, Japan).
Operating conditions: The analytical column was a Capcell Pak 70 C1 UG120 (S-5) packed with C1 methyl-silica, 5 µm dp, 35 mm × 4.6 mm i.d. (Shiseido Co. Inc., Tokyo, Japan) equipped with a guard column (5 mm × 4.6 mm i.d.) containing the same packing material; the isocratic mobile phase was 0.1 mol/L citric acid; the flow-rate was 1.0 mL/min; column temperature was 45 °C; injection volume was 20 µL; and the analytical time was less than 4 min. The PDA detector was operated at 200–380 nm. Monitoring the wavelength was adjusted to 363 nm, which represents an average maximum wavelength for all the target compounds.
Results and Discussion
Sample preparation and optimal HPLC conditions: The present sample preparation method, which is easy-to-use and portable, does not use organic solvents and was rapid and simple with sample preparation times of less than 10 min, resulting in high recovery and repeatability with little differences between each sample.
Considering the packed non-polar sorbents in the columns for RP-HPLC separation, the C1 (methyl-silica) sorbent is the most non-retentive sorbent when retention was based on non-polar interactions alone. To optimize the separation with a 100% aqueous mobile phase and provide a more rapid separation, this study tested six types (Columns A–F, Table 1) of the short packed-C1 columns, which are the highly purified silica-based RP stationary phases.

Table 1
The physical/chemical specifications are listed in Table 1. The author used a citric acid solution as the isocratic aqueous mobile phase and examined mobile phases with molarities of citric acid of ≤ 0.2 mol/L, column temperatures between 25–55 °C, HPLC flow-rates ≥ 0.5 mL/min and HPLC measuring times (retention times) ≤ 20 min: because the HPLC separations were performed serially, the time for each became critical in routine residue monitoring. The short run time not only increased sample throughput for analysis but also affected the method-development time.
An ideal chromatogram with complete separation of OTC, CTC, TC and interfering peaks, their symmetrical natures and their short retention times was obtained using Column A and an isocratic mobile phase of 0.1 mol/L citric acid with a flow-rate of 1.0 mL/min and a column temperature of 45 °C. The HPLC analysis accomplished optimum separation within 4 min and also enabled the multiple sequential injections, without the risks of interfering late-eluting peaks. Under the low-pH environment of the mobile phase and raised column temperature, the short length (35 mm) and carbon contents ≤ 4% in the column were necessary to obtain the findings. Columns B, C and D provided significantly rounded peaks of all the target compounds throughout the examined condition ranges. Column C had difficulty separating OTC, TC and CTC, and the interferences of the resulting sample extract. The target compounds were not eluted from columns E and F.
There was no interference from prawn extract with the elution of OTC, CTC and TC, respectively (Figure 1). In the present HPLC system, separation and quantification were achieved in a mere 5 min for each run without requiring precolumn washing after the analysis.

Figure 1
Method Validation
Table 2 summarizes the main validation parameters. The accuracy, precision, linearity and quantification limit (QL) are well within "acceptable criteria" for the residue analysis that the Codex set-up.20 The decision limits (CCα, α = 5%) and detection capabilities (CCβ, β = 5%) calculated according to the EU regulation decision (2002/657/EU)21 are shown in Table 2.
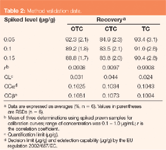
Table 2
Specificity: The application of the proposed procedure to 18 blank samples from different species (Japanese Tiger, Black Tiger, and Shiba Prawn) demonstrated that no interference was present around the retention times for OTC, CTC and TC in any of the samples examined.
Robustness: Some HPLC chromatographic parameters were performed using a spiked (0.15 µg/g of each compound) prawn sample obtained under the established procedure. Changes of ±5% of the flow-rate, the HPLC column temperature and the citric acid concentration of the mobile phase had no effect on the peak areas, whereas the variations in the retention times were obtained with both the flow-rate and the column temperature.
Normal retention times for OTC, TC and CTC were 1.59, 2.24 and 3.63 min, respectively. At +5% the flow-rate, the three retention times were decreased ranging between 1.1 and 3.8% and at –5%, the times were increased, ranging between 4.4–7.1%. By changing the column temperature by +5%, the decreasing retention times obtained were 1.0–7.8%, however, no significant variations were observed with –5%. During these studies, all the target compounds were separated.
Keynotes
Selectivity: The PDA detector is an easy way to confirm peaks and enable the determination of OTC, CTC and TC by their retention times and spectra.
The OTC, CTC and TC peak spectra obtained from the sample were practically identical to those of the standards. Because of the high absorbance of OTC, CTC and TC and the satisfactory purification method, UV detection ( 200–380 nm) is possble at trace levels using PDA.
It is, therefore, instructive to demonstrate purification effectiveness of the sample preparation. The present HPLC system made it unnecessary to use MS, which is very expensive, to analyse the target compounds.
Cost/time performances: The time and budget required for the analysis of one sample were <15 min and roughly €1.25 for each sample respectively. For sequential analyses, a batch of 12 samples can be analysed in less than 80 min.
Application to real prawn samples: Black Tiger Prawns were purchased from a fish market in Osaka, Japan, and used as real prawn samples and analysed using the proposed method. No samples contained detectable concentrations of OTC, CTC and TC. The chromatograms were free from interferences.
Conclusions
This article describes an inexpensive and simple technique of sample preparation followed by RP-HPLC under 100% aqueous conditions for the simultaneous determination of OTC, CTC and TC in prawn. The established procedure, which does not harm the environment or humans, has a short analysis time, is extremely inexpensive and provides reproducible recoveries. The procedure is a useful tool for the routine residue monitoring of OTC, CTC and TC in prawn.
Naoto Furusawa is an associate professor with the Graduate School of Human Life Science, Osaka City University, Osaka 558-8585, Japan.
References
1. http://www.155jvpa.jp/index2.html
2. http://ec.europa.eu/enterprise/pharmaceuticals/mrl/index.html
3. Codex Alimentarius Commission, Joint FAO/WHO Food Standards Programme, 24th Session. Geneva, ALINORM 01/31, (2001).
4. www.jetro.go.jp/jpn/regulations/guidebook/pdf/charge/foodadd2004feb-jp.pdf
5. http://www.agriworld.or.jp/radio/kaigai/genkou/kaigai10_27.html
6. H. Oka et al, J. Chromatogr. A, 882, 109 (2000).
7. WC. Andersen et al., Anal. Chim Acta, 529, 145 (2005).
8. M.J. Schneider et al., J. Chromatogr. B, 6, 8 (2007).
9. A. Pena A et al., J. Agri. Food Chem., 53, 3784 (2005).
10. Y. Wen et al., Talanta, 70, 153 (2006).
11. N. Furusawa, Talanta, 59, 155 (2003).
12. N. Furusawa, Chromatographia, 53, 47 (2001).
13. N. van Eeckhout et al., Rapid Commun. Mass Spectrom., 280 (2000).
14. M. Ishibash, J. Food Hyg. Soc. Jpn., 38, J193 (1997).
15. H. Nakazawa, J. Food Hyg. Soc. Jpn., 38, J193 (1997).
16. R. Malish et al., Dtsch Lebensm-Rundsch., 88, 205 (1992).
17. M.A. Moat, J. AOAC Int., 73, 343 (1990).
18. R.T. Anastas and J.C. Warner, Green Chemistry — Theory and Practice (Oxford University Press, UK, 1998).
19. T. Yoshimura et al., Green Chemistry — Aim for the zero emission-chemicals, (Sankyo Publishing Co. Ltd Press, Tokyo, Japan, 2001).
20. Codex Alimentarius Commission, "Joint FAO/WHO Food Standards Program, Residues of Veterinary Drugs in Food, Vol. 3", 2nd ed. (Codex Alimentarius Commission, Roma, Italy, 1993).
21. EC Decision 2002/657, Off J Eur Commun L221/8 (2002).

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.












